|
|
|
Indian Pediatr 2016;53:
575-581 |
 |
Expanded Indian National Rotavirus
Surveillance Network in the Context of Rotavirus Vaccine
Introduction
|
|
Sanjay Mehendale, S Venkatasubramanian, CP Girish
Kumar, *Gagandeep
Kang,
#MD Gupte
and #Rashmi Arora
From National Institute of Epidemiology, Chennai;
*Christian Medical College, Vellore; and #Indian Council of Medical
Research, New Delhi; India.
Correspondence to: Dr. Sanjay Mehendale, Director,
National Institute of Epidemiology, Indian Council of Medical Research,
II Main Road, TNHB, Ayapakkam, Chennai – 600077, India.
Email:
[email protected]
Received: April 24, 2015;
Initial review: July 08, 2015;
Accepted: April 09, 2016.
|
Objective: To extend a
nation-wide rotavirus surveillance network in India, and to generate
geographically representative data on rotaviral disease burden and
prevalent strains.
Design: Hospital-based
surveillance.
Setting: A comprehensive
multicenter, multi-state hospital based surveillance network was
established in a phased manner involving 28 hospital sites across 17
states and two union territories in India.
Patients: Cases of acute diarrhea
among children below 5 years of age admitted in the participating
hospitals.
Results: During the 28-month
study period between September 2012 and December 2014, 11898 children
were enrolled and stool samples from 10207 children admitted with acute
diarrhea were tested; 39.6% were positive for rotavirus. Highest
positivity was seen in Tanda (60.4%) and Bhubaneswar (60.4%) followed by
Midnapore (59.5%). Rotavirus infection was seen more among children aged
below 2 years with highest (46.7%) positivity in the age group of 12-23
months. Cooler months of September – February accounted for most of the
rotavirus-associated gastroenteritis, with highest prevalence seen
during December – February (56.4%). 64% of rotavirus-infected children
had severe to very severe disease. G1 P[8] was the predominant rotavirus
strain (62.7%) during the surveillance period.
Conclusions: The surveillance
data highlights the high rotaviral disease burden in India. The network
will continue to be a platform for monitoring the impact of the vaccine.
Keywords: Epidemiology; Rotavirus diarrhea,
Vaccine.
|
|
Diarrheal diseases account for 1 in 10 child
deaths worldwide, making diarrhea the second leading cause of deaths
among children under the age of 5 years [1]. Rotavirus infections are
the most common cause of severe gastroenteritis in children below 5
years of age worldwide and account for 5% of all deaths among children
in this age group [2]. Nearly 453,000 (420,000 – 494,000) child deaths
were estimated to have occurred globally during 2008 due to rotavirus
infection with 22% in children below five years of age in India alone
[3]. Recent estimates have shown that about 872,000 hospitalizations and
78,500 deaths occur due to rotavirus infections annually in India [4].
Several studies on rotavirus disease have been
conducted across different parts of India but due to differences in
study design, operational definitions, populations examined, recruitment
strategies, and detection systems used [5]; information on the national
level on rotavirus diarrhea disease burden is not available.
The ‘National Rotavirus Surveillance Network’ (NRSN)
was established in December 2005 by the Indian Council of Medical
Research (ICMR) to evolve a sustainable surveillance platform to
estimate and monitor the disease burden in children under 5 years of age
hospitalized for diarrhea [5,6]. In order to make informed decisions
regarding possible phased introduction of rotavirus vaccine in the
country as part of the national immunization program, the surveillance
network was expected to generate data on rotavirus disease burden and
monitoring trends over time across the country. Another objective was to
generate data on molecular epidemiology of rotavirus in India. This
network generated valuable data for the period of 2005-2009. The first
round of the NRSN (2005- 2009) was initiated with four laboratories and
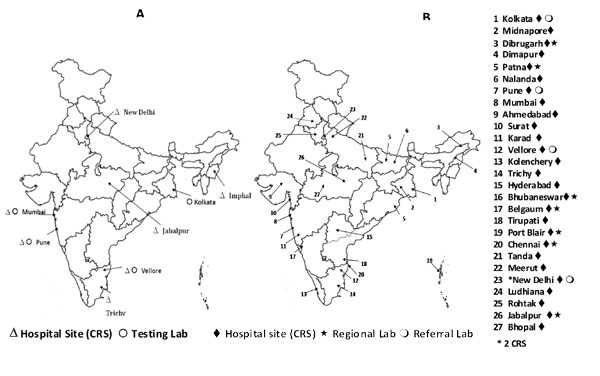
ten hospitals in seven different regions of India (Fig. 1).
The detailed results have been published earlier [5,6]. The analysis of
surveillance data involving over 7000 children demonstrated both the
high prevalence of rotavirus disease in India as well as the circulation
of a diverse range of rotavirus strains [6]. Another notable finding was
the emergence of G12 strains, particularly G12 P[6] strains, in both the
Western and Northern region [5,6].
 |
|
Fig. 1 Hospital sites and testing
laboratories (a) 2005 -2009 (b) 2012-2016.
|
In November 2011, the expert advisory group set up by
ICMR recommended expansion to include more clinical surveillance sites
in hospitals and testing laboratories network to have more complete
regional representation of the country. Following this recommendation,
the NRSN was expanded to generate pan-India data on rotavirus disease
burden. The present paper describes the process, methodology and
framework of the expanded network (2012 onwards), and highlights the key
findings and lessons learnt in this endeavor. Detailed analysis of the
surveillance data is published in a separate paper.
Methods
The Expanded National Rotavirus Surveillance Network
(ENRSN) was planned to conduct hospital-based surveillance for rotavirus
from 2009 onwards across the country. The network includes four Referral
and seven Regional laboratories in Southern, Western, Northern and
Eastern/ North-Eastern regions of the country, and 28 clinical
recruitment sites (CRS) of hospitals in 17 states and two Union
Territories with inpatient pediatric facilities that contribute clinical
data and samples. The present ENRSN platform has been built with three
phases of expansion in different parts of India. In Phase-1, the
surveillance was initiated in September 2012 at 8 sites viz.
Vellore (Tamil Nadu), Trichy (Tamil Nadu), Hyderabad (Telangana),
Tirupati (Andhra Pradesh), Ludhiana (Punjab), Kolenchery (Kerala), New
Delhi and Port Blair (Andaman & Nicobar). In September-October 2013, CRS
in 12 cities viz. Kolkata (West Bengal), Midnapore (West Bengal),
Pune (Maharasthra), Mumbai (Maharasthra), Karad (Maharasthra), Tanda
(Himachal Pradesh), Meerut (Uttar Pradesh), New Delhi, Rohtak (Haryana),
Dibrugarh (Assam), Dimapur (Nagaland), Belgaum (Karnataka), Surat
(Gujarat) and Ahmedabad (Gujarat) initiated surveillance as part of the
Phase-2 expansion. In Phase-3, additional sites [Patna (Bihar), Nalanda
(Bihar), Bhubaneswar (Odisha), Chennai (Tamil Nadu), Jabalpur (Madhya
Pradesh) and Bhopal (Madhya Pradesh)] started enrolling children in
surveillance in July - August 2014. The network has been formalized by
signing Memorandum of Understanding (MoUs) between each CRS, affiliated
referral or regional laboratory and the coordinating center. The list of
participating investigators and centers is provided as Annexure
1.
The Epidemiology and Communicable Diseases (ECD)
Division of ICMR is responsible for administrative coordination of the
network, providing funding and leadership to the network. The National
Institute of Epidemiology is the coordinating centers for this
surveillance network involving 28 CRS across the country, and is
responsible for selecting and initiating CRS, training in field
component of the study protocol and data management. Its additional
responsibility includes coordination, monitoring and evaluation as well
as quality control of the clinical component, sample transfers, data
management and analysis. Christian Medical College (CMC), Vellore is the
coordinating laboratory that conducts training of laboratory personnel
for laboratory procedures, designs and implements laboratory protocols
and ensures laboratory quality management at all levels.
Clinical/Field Component
Site selection and support: Clinical recruitment
sites (CRS) were identified by the National Institute of Epidemiology
(NIE) with the help of partner institutes (referral and regional
laboratories) in the respective regions viz. South, West, North
and East/ North-East.
Establishing surveillance: The sanctioning and
funding of each CRS and partner laboratories involved completion of
specific documentation and submission to ICMR. The documentation process
included signing of a MoU/ undertaking and obtaining clearance from the
Ethics committees of the concerned hospitals / institutes.
Patient enrolment: All cases of acute diarrhea ( ³3
loose stools in a 24 hour period) and of duration not greater than 5
days among children aged (0-59 months) admitted to the CRS for
supervised oral or intravenous rehydration were considered for
enrollment under ENRSN. Eligible children were enrolled after obtaining
written informed consent from the parents/guardians. Each CRS enrolled
children throughout the year, collected clinical data including history
of rotavirus vaccination on a standardized clinical recruitment
form/case report form (CRF), and also collected stool samples. Records
such as date of admission for diarrhea management and enrolment were
recorded in a diarrhea hospitalization logbook maintained in each CRS.
Sample collection: Whole stool specimen (~5ml)
was collected from each child enrolled in the study and transported
within 2 hours to the testing laboratory or stored at 4 0C
until transportation. Samples stored at 40C
after collection were transported in boxes with ice packs at weekly or
fortnightly intervals to the testing laboratory.
Laboratory Testing
Stool samples were subjected to rotavirus screening
using a commercial enzyme immunoassay (Premier
Rotaclone, Meridian Biosciences) and genotyping
for VP7 (G-typing) and VP4 (P-typing) by Reverse-transcription
polymerase chain reaction (RT-PCR) [5]. Results of the laboratory
analyses were recorded in a laboratory log book maintained in each
testing laboratory.
Seven regional laboratories carried out ELISA for
rotavirus antigen detection on stool samples collected at affiliated
CRS. The regional laboratories were also responsible for entry of both
clinical and laboratory data. Four referral laboratories carried out
ELISA for screening on samples collected at CRS directly assigned to
them, PCR for genotype characterization (including a subset of rotavirus
positive samples from affiliated regional laboratories), and also
completed data entry. Referral/ Regional Laboratories submitted monthly
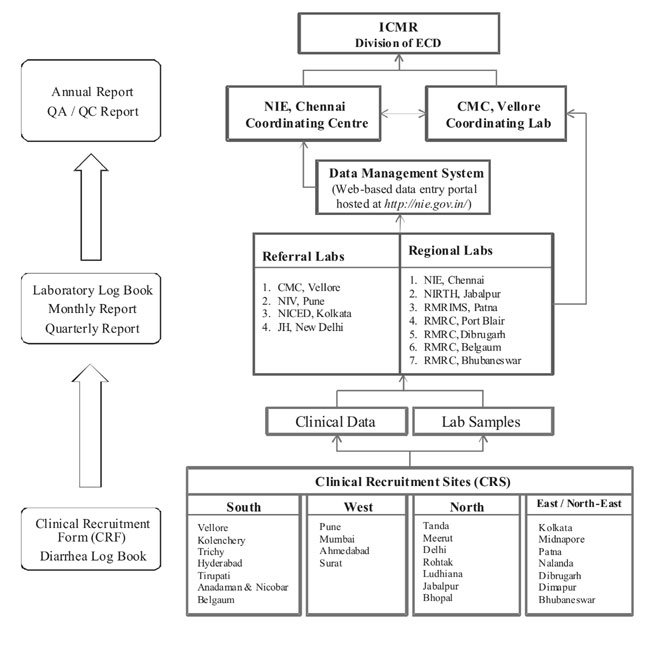
and quarterly reports. The organizational framework and data flow in the
network is described in Fig. 2.
 |
|
Fig. 2 Sample and data flow in
National Rotavirus Surveillance Network.
|
Training: Each referral and regional laboratory
conducted hands-on training for staff recruited for the surveillance
activity covering the three main components of the ENRSN program;
namely, clinical, sample management, laboratory analysis and data entry.
Quality assurance: The coordinating laboratory at
CMC, Vellore in coordination with NIE sent pretested panels of positive
and negative samples to all the participating laboratories annually.
Similarly CMC received set of ten consecutively tested samples for
retesting from all the laboratories bi-annually. A panel of public
health and child health experts constituted by ICMR in coordination with
NIE carried out periodic monitoring visits to all levels of the network.
Data management: In the expanded round of NRSN a
comprehensive, web-based data management system was introduced by NIE.
The features of this system include double data entry, validation,
innovative data matching interface, record level blocking and efficient
data export to NIE, the Coordinating Center for ENRSN. The
details of the data management framework have been described elsewhere
[8]. Regional and referral laboratories were responsible for the data
entry of the CRFs. Diarrheal disease severity was calculated using the
Vesikari score [5]. Data validation and analysis was carried out at NIE.
Statistical analysis: Data were analyzed to
assess the proportions of rotavirus-positive cases in terms of
demographic factors, seasons, regions, disease severity and genotype
distribution using SPSS 17.0.
Results
During the 28-month study period from September 2012
to December 2014, a total of 14315 children with acute gastroenteritis
were admitted to the participating hospitals and 11898 children were
enrolled in to the surveillance. Among the 10207 children from whom
stool samples were collected and analyzed, 39.6% were positive for
rotavirus (Table I). The highest rotavirus positivity was
seen in Tanda (60.4%) and Bhubaneswar (60.4%), followed by Midnapore
(59.5%). The lowest prevalence was observed in Nalanda (3.6%) and Patna
(7.3%). Overall, most of the surveillance sites registered high
proportion of rotavirus positivity.
TABLE I Regional and Site-wise Rotavirus Positivity Rates of Children Hospitalized with Acute Gastroenteritis
|
Region |
CRS |
Admitted/Enrolled* |
Number of Samples Tested# |
Rotavirus N(%) Positive#
|
|
East |
Kolkata |
660/461 |
431 |
228 (52.9) |
|
Midnapore |
918/602 |
592 |
352 (59.5) |
|
Bhubaneswar |
710/411 |
268 |
162(60.4) |
|
Dibrugarh |
356/356 |
279 |
102(36.6) |
|
Dimapur |
326/326 |
260 |
117(45.0) |
|
Patna |
528/309 |
301 |
22(7.3) |
|
Nalanda |
244/230 |
169 |
6(3.6) |
|
Total |
3742/2695 |
2300 |
989(43.0) |
|
West |
Pune |
532/469 |
368 |
204(55.4) |
|
Mumbai |
453/415 |
369 |
133(36.0) |
|
Ahmedabad |
306/304 |
231 |
58(25.1) |
|
Surat |
540/508 |
424 |
169(39.9) |
|
Karad |
447/433 |
363 |
125(34.4) |
|
Total |
2278/2129 |
1755 |
689(39.3) |
|
South |
Vellore |
1088/957 |
789 |
240(30.4) |
|
Kolenchery |
790/709 |
529 |
239(45.2) |
|
Trichy |
331/324 |
282 |
141(50.0) |
|
Hyderabad |
756/410 |
388 |
93(24.0) |
|
Tirupati |
447/444 |
433 |
117(27.0) |
|
Belgaum |
290/280 |
255 |
80(31.4) |
|
Port Blair |
966/966 |
910 |
385(42.3) |
|
Chennai |
320/281 |
226 |
64(28.3) |
|
Total |
4988/4371 |
3812 |
1359(35.7) |
|
North |
Tanda |
352/272 |
245 |
148(60.4) |
|
Meerut |
332/321 |
282 |
56 (19.9) |
|
Delhi |
1488/1209 |
1070 |
550(51.4) |
|
Rohtak |
501/269 |
246 |
86(35.0) |
|
Ludhiana |
479/477 |
384 |
136(35.4) |
|
Jabalpur |
79/79 |
65 |
13(20.0) |
|
Bhopal |
76/76 |
48 |
16(33.3) |
|
Total |
3307/2703 |
2340 |
1005(42.9) |
|
Total |
14315/11898 |
10207 |
4042(39.6) |
|
*Data from hospital records; # data from
validated data analysis. |
Prevalence of rotavirus positivity in gastroenteritis
was 38.1% (1890/4962) among children below one year of age; whereas the
highest rotavirus disease burden was seen among children between 12-23
months (46.7%; 1514/3244). There was no significant difference in
rotavirus positivity rates by gender. Pooled data analysis of seasonal
prevalence showed that rotavirus infections were seen more commonly
during the cooler months (September – February). The highest prevalence
was observed during December – February (56.4%; 1668/2959) and lowest
during June – August (20.6%; 461/2239). Diarrheal disease severity
analysis among rotavirus-infected children revealed that 64% (2585/4042)
of rotavirus infected children had severe or very severe disease.
Genotype analysis of a subset of rotavirus positive samples showed that
G1 P [8] was the predominant strain (62.7%; 1579/ 2519). Clear history
of rotavirus vaccination was available for only 3.3% (340/10206) of
children.
Discussion
The prevalence of rotaviral diarrhea among Indian
children aged less than 5 years included in ENRSN (September 2012 to
December 2014) was 39.6%. This is in conformity with the findings of the
earlier round of NRSN (2005-2009) [5,6]. Inclusion of fewer hospital
sites in the first phase of surveillance (2005-2009) limited the
generalizability of the surveillance findings due to limited
geographical representation.
The notable improvements in ENRSN included more
involvement of government medical colleges / facilities which has led to
capacity building in these centers for undertaking surveillance
activities. All the project staff has been trained, and they are
following identical protocols for enrolment, sample collection, clinical
and laboratory procedures, and data management. The CRF has been modeled
on the online data entry platform in the current round of surveillance.
The CRF has been made simpler and more user friendly and data on
rotavirus vaccination history and dosage is being systematically
collected. Through regular supervision and monitoring, focus is being
given on quality management across the network.
The limitation associated with this hospital-based
surveillance program was its inability to generate community-level
disease burden data. Moreover, despite establishing a large network of
28 hospital sites across the country, some states are still outside the
coverage of the network.
Only a small proportion of children attending various
hospitals in the network had history of rotavirus vaccination.
Commercial rotavirus vaccines are available in India since 2007-2008,
and rotavirus vaccine is part of the optional vaccines recommended by
the Indian Academy of Pediatrics. The high price of these vaccines drove
them out of reach of the common man restricting their usage to a small
proportion of children from affluent sections of Indian society. This
may be one reason why a substantially large population of children
continued to get infected and consequently developed severe diarrhea. It
is anticipated that the launch of indigenous rotavirus vaccine in the
national immunization program may result in substantial reduction in the
morbidity and mortality associated with rotaviral gastroenteritis in the
country, especially in areas with high rotavirus diarrhea burden.
Possible linkages between ENRSN and other
surveillance networks in India like hospital-based sentinel surveillance
of bacterial meningitis would result in better implementation and
coordination of one or more surveillance programs in the same hospital
setup. The network platform at various levels viz. district,
state or regional levels, could cater to hypothesis generation for
commissioning satellite studies for locally relevant issues. Besides
surveillance for rotavirus, the ICMR supported ENRSN also offers
appropriate infrastructure to conduct facility-based surveillance for
other related pathogens and supplementary studies such as economic
analysis and cost-effectiveness studies related to vaccine introduction.
In future, ENRSN will provide a unique opportunity
for assessment of impact of vaccination on rotavirus disease in
post-rotavirus vaccine introduction in India in a phased manner by
estimating rotavirus disease burden, severity of disease, circulating
genotypes as well as vaccine related severe adverse events like
intussusception. A pilot retrospective case-record based survey for
intussusception has been carried out involving several hospitals in
Chennai (unpublished report), and efforts are on to conduct a larger
multi-site survey and surveillance for intussusception using the ENRSN
network platform. The surveillance is currently ongoing in 19 states/
UTs and newer hospital sites in states outside the coverage of the
network will also be considered for inclusion to generate micro-level
data on rotaviral disease burden in future.
Contributors: SMM: Network coordination,
manuscript conceptualization and writing; SV and CPGK: contributed to
network coordination, and manuscript writing; GK: Coordinated laboratory
activities in the network and provided intellectual inputs for
manuscript writing; MDG and RA: Coordination at ICMR level and provided
intellectual inputs to for manuscript writing. All authors approved the
final manuscript.
Funding: Indian Council of Medical
Research; Competing interests: None stated.
Annexure 1
Participating investigators of National Rotavirus
Surveillance Network [2012 – 2016]
Agarwal A (Netaji SC Bose Medical College & Hospital,
Jabalpur); Aneja S (Kalawati Saran Children Hospital (LHMC) New Delhi);
Anna Simon (Christian Medical College, Vellore); Aundhakar SC (Krishna
Institute of Medical Sciences, Karad); Babji S (Medical College, Vellore
and Infant Jesus Hospital, Trichy); Bavdekar A (KEM Hospital, Pune);
Baveja. S (Lokmanya Tilak Municipal GH & Medical College, Mumbai); Bhat
J (National Institute for Research in Tribal Health, Jabalpur); Biswas D
(Assam Medical College & Hospital, Dibrugarh); Bora CJ (Assam Medical
College & Hospital, Dibrugarh); Borkakoty B (Regional Medical Research
Centre, Dibrugarh); Chatterjee S (Midnapore Medical College, Midnapore);
Chaudhary S (Dr. Rajendra Prasad Government Medical College, Tanda);
Chawla-Sarkar M (National Institute of Cholera and Enteric Diseases,
Kolkata); Chitambar SD (National Institute of Virology, Pune); Das P
(Rajendra Memorial Research Institute of Medical Sciences, Patna); Das
VNR (Rajendra Memorial Research Institute of Medical Sciences, Patna);
Desai K (Surat Municipal Institution of Medical Education & Research,
Surat); Dhongade R (Sant Dnyaneshwar Medical Education & Research
Centre, Pune); Dwibedi. B (Regional Medical Research Centre,
Bhubaneswar); Dwivedi R (Kamla Nehru Hospital, Gandhi Medical College,
Bhopal); Dzuvichu K (District Hospital, Nagaland); Ganguly N (Institute
of Child Health, Kolkata); Gathwala G (Pt. B.D.Sharma PGIMS, Rohtak);
Ghosh C (Midnapore Medical College, Midnapore);Gopalkrishna V (National
Institute of Virology, Pune); Gupta DS (Surat Municipal Institution of
Medical Education & Research, Surat); Jadhav AR (Lokmanya Tilak
Municipal GH & Medical College, Mumbai); Jali S (Jawaharlal Nehru
Medical College, Belgaum); Kalrao VR (Bharati Vidyapeeth Medical
College, Pune); Kar SK (Regional Medical Research Centre, Bhubaneswar);
Khuntia HK (Regional Medical Research Centre, Bhubaneswar); Kumar P (Kalawati
Saran Children Hospital, New Delhi); Kumar S (National Institute for
Research in Tribal Health, Jabalpur); Kumar SS (Pragna Children’s
Hospital, Hyderabad); Lal BG (BJR Hospital, Port Blair); Manglani M (Lokmanya
Tilak Municipal GH & Medical College, Mumbai); Manohar B (Sri
Venkateswara Institute of Medical Sciences, Tirupati); Mathew A (St.
Stephen’s Hospital, Delhi); Mathew MA (MOSC Medical College, Kolenchery);
Mehariya KM (BJ Medical College & Civil Hospital, Ahmedabad); Mishra SK
(Capital Hospital, Bhubaneswar); Narayanan SA (Institute of Child Health
& Hospital for Children, Chennai); Niyogi P (Institute of Child Health,
Kolkata); Panda S (National Institute of Cholera and Enteric Diseases,
Kolkata); Pandey K (Rajendra Memorial Research Institute of Medical
Sciences, Patna); Patankar M (BJ Medical College & Civil Hospital,
Ahmedabad); Purani CS (BJ Medical College & Civil Hospital, Ahmedabad);
Ray P (Jamia Hamdard, New Delhi); Roy S (Regional Medical Research
Centre, Belgaum); Sahoo GC (Rajendra Memorial Research Institute of
Medical Sciences, Patna); Singh N (National Institute for Research in
Tribal Health, Jabalpur); Singh P (Surat Municipal Institution of
Medical Education & Research, Surat); Singh T (Christian Medical
College, Ludhiana); Sundari S (Institute of Child Health & Hospital for
Children, Chennai); Temsu T (District Hospital, Nagaland); Thakur AK (Nalanda
Medical College & Hospital, Nalanda); Topno RK (Rajendra Memorial
Research Institute of Medical Sciences, Patna); Upadhyay A (Lala Lajpat
Rai Memorial Medical College, Meerut); Utpalkant Singh (Child Care
Centre, Patna); Vijayachari P (Regional Medical Research Centre, Port
Blair).
.
|
What is Already Known?
•
First phase of rotavirus
surveillance in India (2005-09) documented the rotavirus disease
burden at selective sites in the country.
What This Study Adds?
•
Expanded National Rotavirus Surveillance Network validates
the findings of the previous surveillance, highlights the high
prevalence (39.6%) of rotavirus disease burden across the
country, and documents baseline national level data at the point
of rollout of rotavirus vaccine in the national immunization
program.
|
References
1. Centers for Disease Control and Prevention. Global
Diarrhea Burden. Available from: www.cdc.gov/healthy
water/global/diarrhea-burden.html#one. Accessed March 21, 2015.
2. Tate JE, Chitambar S, Esposito DH, Sarkar R,
Gladstone B, Ramani S, et al. Disease and economic burden of
rotavirus diarrhea in India. Vaccine. 2009;27:F18-24.
3. World Health Organization. Rotavirus Mortality
Estimates. Available from:
www.who.int/immunization/monitoring_surveillance/burden/estimates/rotavirus/en.
Accessed March 21, 2015.
4. John J, Sarkar R, Muliyil J, Bhandari N, Bhan MK,
Kang G. Rotavirus gastroenteritis in India, 2011-2013: Revised estimates
of disease burden and potential impact of vaccines. Vaccine.
2014;32:A5-9.
5. Kang G, Arora R, Chitambar SD, Deshpande J, Gupte
MD, Kulkarni M, et al. Multicenter, hospital-based surveillance
of rotavirus disease and strains among Indian children aged <5 years. J
Infect Dis.2009;200: S147–S53.
6. Kang G, Desai R, Arora R, Chitamabar S, Naik TN,
Krishnan T, et al. Diversity of circulating rotavirus strains in
children hospitalized with diarrhea in India, 2005–2009. Vaccine.
2013;31:2879-83.
7. Girish Kumar CP, Venkatasubramanian S, Kang G,
Arora R, Mehendale S, for the National Rotavirus Surveillance Network.
Profile and trends of rotavirus gastroenteritis in under five
children in India (2012 – 2014): preliminary report of the Indian
National Rotavirus Surveillance Network. Indian Pediatr. 2016.53:
8. Ravi M, Venkatasubramanian S, Girish Kumar CP,
Arora R, Mehendale S. Online Data Management System for National
Rotavirus Hospital Based Surveillance Network (NRSN) 2013. Available at
www.nie.gov.in/ezine/ezine2.3/technofile.php#link1. Accessed
March 21, 2015.
|
|
|
 |
|

