In 1973, Ruth Bishop and colleagues published a
paper in the Lancet describing virus particles in epithelial cells of
the duodenal mucosa of children with acute non-bacterial gastroenteritis
[1]. Two years later, Ian Holmes, the electron microscopist – who had
seen the round-wheel shaped structures in the samples that Bishop
provided – visited Southern India and taught Minnie Mathan at the
Christian Medical College, Vellore how to recognize these distinctive
viruses. The first paper on rotavirus from India was published in 1977
[2] that described the virus to be associated with 26% of severe
gastroenteritis. Shortly thereafter, Dr. Panicker in Calicut (as it was
then known), contacted Dr. Mathan to analyze samples from an outbreak of
gastroenteritis, and their joint work demonstrated that rotavirus was
the cause of the outbreak and subsequently that rotavirus disease was
seasonal [3].
Scientists learnt that it was possible to distinguish
rotaviruses based on the patterns of migration of the 11 segments of
double-stranded RNA. Electropherotyping methods were established that
showed different circulating types of rotavirus, with variations by
location. Subsequently, enzyme immunoassays become available, and
several researchers in India began to identify rotavirus infections not
only in children with acute diarrhea, but also in animals [4,5]. Bhan,
et al. [6] from All India Institute of Medical Sciences (New
Delhi) showed that a large proportion of neonates in their nursery were
asymptomatically infected with rotavirus. When these babies were
followed up over time, it was shown that these children were protected
from severe rotavirus gastroenteritis, and that the strains isolated
from these children were all similar based on electropherotyping [6].
Dr. Bhan, who later became the Secretary of the Department of
Biotechnology (DBT; Government of India), collaborated with Roger Glass,
whom he had met when Dr. Glass had worked in Bangladesh. Dr. Glass went
on to the Centers of Disease Control and Prevention in Atlanta, and
supported the characterization of the 116E neonatal strain that had been
isolated in AIIMS, by Dr Bimal Das. His work, based on sequencing of the
strain, showed that the strain was unusual, in belonging to the G9P[11]
serotype, because most strains detected up to that time from humans had
been G1-4 and in being a natural reassortant strain, carrying the P[11]
gene of bovine origin [7]. Further studies explored why neonates were
infected when mothers had transferred anti-rotavirus antibodies to their
infants. It was shown that the infected children did mount an antibody
response, and it was postulated that the presence of the bovine capsid
protein allowed the children to get infected even though transplacental
or breast milk antibodies were received from the mother [8].
A similar story emerged in Bangalore, where C Durga
Rao and his colleagues identified a strain that asymptomatically
infected neonates resulting in subsequent decreases in rotavirus
infection and disease. The strain, called I321, was also a bovine human
reassortant, but unlike 116E, which has only one bovine gene, it
consisted of mainly bovine genes [9].
While these studies were being done, through the
1980s and early 1990s, various enzyme immunoassays and electrophoresis
techniques were used to identify rotaviruses from children in
out-patient and in-patient settings, and wherever studies were done,
rotaviruses were associated with a significant proportion of acute
diarrheal disease, up to 20-50% with winter peaks, particularly in the
North [10]. The enzyme immunoassay kits were expensive and the National
Institute of Virology developed reagents for a similar test for
rotavirus [11], but it was not widely used, because testing for
rotavirus in routine practice was non-existent. Specific sera for typing
of the two outer capsid proteins became available through international
collaborations and the diversity of rotaviruses in India and the change
in strains was increasingly evident. When polymerase chain reaction
(PCR)-based techniques were well established in the 1990s, they
confirmed the finding of high diversity and the occurrence of unusual
strains, possibly due to zoonotic infections [12].
In parallel to the several small surveillance
studies, the two neonatal strains of rotavirus that had been identified
in Delhi and Bangalore were adapted to cell culture and grown to make
vaccine candidates. The DBT (India) and the National Institutes of
Health (US) supported rotavirus vaccine development through the Indo-US
Vaccine Action Program (VAP) that was established in the 1980s through
several grants. In the late 1990s, the VAP decided to support a new
company, Bharat Biotech International Limited, to take the development
of 116E and I321 vaccines forward. Initial phase I testing had been
conducted in the US with support from the CDC and NIH, but the studies
were repeated in India and extended into phase II [9]. In phase II, the
I321 strain was found to be less immunogenic with only 30% of children
seroconverting, whereas the 116E strain seroconverted more than 80% of
children, and hence only the 116E strain was taken forward into phase
III. Other studies in neonates had shown that strains, that resembled
I321, infected children in Vellore, and that these children were not
protected from subsequent rotavirus infection or diarrhea [13]. While
the indigenous vaccine candidate was undergoing clinical testing, the
Indian Council for Medical Research (ICMR) decided to make a large
investment in rotavirus surveillance and established a multi-site
network, which unlike several previous studies that had all differed in
study design and diagnostic approaches, used similar methods for
recruitment and testing. This standardized approach revealed that unlike
previous studies which had estimated that rotavirus caused about 20% of
hospitalized gastroenteritis, the proportion that were testing positive
was closer to 40% [12]. In addition to the studies focused on burden of
disease and vaccination, Indian researchers initiated more basic studies
on the biology of rotavirus – studying structure and function of
rotaviral proteins, thus complementing the work that is being conducted
in other settings [14,15].
Despite the basic research and the multitude of
hospital-based studies, there have been very few community-based studies
on rotavirus in India. The largest birth cohort study to evaluate
rotavirus infection was conducted in Vellore between 2001 and 2006 [16,
17]. This study showed that unlike the previous birth cohort studies in
other parts of the world – although rotavirus infection was common and
rotavirus was the most important pathogen causing diarrhea in the
community – the protection afforded by prior rotavirus infection was
less than that seen in other birth cohorts [16]. This led to the
question of how well vaccines would work, and modelling studies based on
the Vellore data estimated a protection of about 50% in disadvantaged
populations [18].
While the indigenous vaccine candidate was in phase
II and III studies, the two internationally licensed vaccines underwent
immunogenicity bridging studies at multiple sites in India. Based on 58%
immunogenicity for Rotarix and 83% for Rotateq – but by different ways
of assessing immunogenicity – Rotarix and Rotateq, were licensed [19,
20], and used in the private market, with the Indian Academy of
Pediatrics, reviewing their performance and making recommendations for
their use [21]. In 2014, the results of the efficacy trial with 116E
became available, and at 55% efficacy, the performance of this vaccine
was comparable to that of Rotarix and Rotateq in Africa and other
countries in Asia [22]. This was despite the fact that the very close
monitoring and early treatment of children in the efficacy trial
considerably reduced the incidence of severe disease.
In parallel with the vaccine testing, a number of
studies estimated the burden of disease in India and the
cost-effectiveness of rotavirus vaccines, and all studies demonstrated
that in India, the vaccines would be cost-effective at the price at
which vaccines were available for the Indian private and public markets
[23,24]. India’s birth cohort of 27 million is the largest in the world,
and unfortunately even though the number of diarrheal deaths is
decreasing rapidly, the number of deaths attributed to rotavirus is
numerically the largest for any country. Given that mortality due to
diarrheal disease is decreasing with access to care, rehydration and
better nutrition, the impact of vaccines should be measured not only as
reduction in mortality but also in averted hospitalizations, as
emphasized in an editorial in this issue [25]. There is also a need to
revisit cost-effectiveness, since mortality has decreased and costing
studies which informed earlier estimates were collected a decade ago and
excluded costs in children admitted with gastroenteritis who required
higher levels of care, which are now available, along with limited more
recent estimates [26,27].
The several studies by the ICMR and DBT as well as
academic researchers in India over decades, resulted in a situation that
when affordable rotavirus vaccines became available for the national
immunization program, the evidence base for vaccine introduction and the
cost utility of rotavirus vaccines already existed, and it was possible
for the National Technical Advisory Group on Immuni-zation to recommend
to the Ministry of Health and Family Welfare that the vaccine should be
introduced for the children who need it the most. The recommendation was
accepted and a phased introduction began in 2016 with Odisha, Andhra
Pradesh, Haryana and Himachal Pradesh, but will roll out nation-wide as
supply becomes available for the rest of India.
Other than for polio, which was a global eradication
effort, and hence different from rotavirus, there have been few
systematic efforts to assess the impact of a newly introduced vaccine in
India. In countries where vaccines have been introduced nation-wide,
there have been remarkable effects of reduction in severe rotavirus
gastroenteritis, all-cause gastroenteritis and all-cause gastroenteritis
mortality as well as reductions in gastroenteritis in unvaccinated age
groups, indicating a herd effect [28]. Such studies are planned for
India which will assess the effectiveness of the vaccine in routine use
as well as monitor its safety [25,29]. Several concerns have been raised
in the media about the safety of rotavirus vaccines and the potential
for intussusception. Both Rotarix and Rotateq have been associated with
a small increased risk where they have been given to several hundreds of
thousands of children [28]. Rotavac, the vaccine that will, at least,
initially, be used in the public health immunization system in India,
has not been tested in such large numbers, and while the studies
conducted so far have shown no risk, there need to be continued
monitoring both through the post-marketing surveillance required by the
Drugs Controller General of India as well as in the public health
immunization system.
The ICMR has been preparing for the monitoring of
impact through expanded surveillance, which shows that the burden
continues to be high [30,31], and similar studies have also been
conducted by other researchers across India [32-35]. While the
epidemiologic and vaccine studies were conducted during the past decade,
there were also efforts to understand the basis of the immune response
to rotavirus and rotavirus vaccines [36, 37] and the reasons why
rotavirus vaccination efficacy was less in developed than in developing
countries. Several reasons have been proposed, including high levels of
maternal antibodies, environmental enteropathy, and malnutrition or
micronutrient deficiencies. Studies are being conducted on approaches to
improve performance of vaccines, but with little success so far [38,
39]. One question that remains unanswered is how well rotavirus vaccines
perform in children of upper socio-economic status in India, and such a
study has never been done.
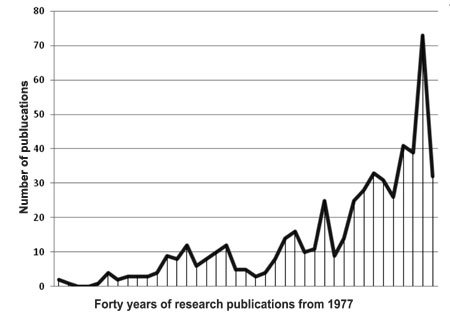 |
|
Fig. 1 Peer-reviewed publications on
rotavirus from India.
|
Other companies in India are also working on
rotavirus vaccines, with Serum Institute of India, Shantha Biotechnics
and Hilleman Laboratories all having rotavirus vaccine programs at
various stages of development. Overall, rotavirus has been one vaccine
preventable disease where India has kept pace with the rest of the world
in conducting comprehensive research, with over 500 studies resulting in
publications in peer-reviewed journals (Fig. 1). We have
now developed at least one indigenous vaccine, and whether it is this
vaccine or others that are used, we should ensure that we continue to
conduct appropriate research to monitor this important cause of
childhood gastroenteritis, its treatment and prevention.
1. Bishop RF, Davidson GP, Holmes IH, Ruck BJ.
Detection of a new virus by electron microscopy of faecal extracts from
children with acute gastroenteritis. Lancet. 1974;1:149-51.
2. Maiya PP, Pereira SM, Mathan M, Bhat P, Albert MJ,
Baker SJ. Aetiology of acute gastroenteritis in infancy and early
childhood in southern India. Arch Dis Child. 1977;52:482-5.
3. Paniker CK, Mathew S, Dharmarajan R, Mathan MM,
Mathan VI. Epidemic gastroenteritis in children associated with
rotavirus infection. Indian J Med Res. 1977;66:525-9.
4. Hrdy DB. Rotavirus antibodies in hanuman langurs (Presbytis
entellus). J Med Primatol. 1982;11:35-8.
5. Singh A, Pandey R. Analysis of electrophoretypes
of rotavirus from diarrhoeic faeces of neonatal buffalo calves in India.
Acta Virol. 1988;32:156-9.
6. Bhan MK, Lew JF, Sazawal S, Das BK, Gentsch JR,
Glass RI. Protection conferred by neonatal rotavirus infection against
subsequent rotavirus diarrhea. J Infect Dis. 1993;168:282-7.
7. Cunliffe NA, Das BK, Ramachandran M, Bhan MK,
Glass RI, Gentsch JR. Sequence analysis demonstrates that VP6, NSP1 and
NSP4 genes of Indian neonatal rotavirus strain 116E are of human origin.
Virus Genes. 1997;15:39-44.
8. Ramachandran M, Vij A, Kumar R, Das BK, Gentsch
JR, Bhan MK, et al. Lack of maternal antibodies to P serotypes
may predispose neonates to infections with unusual rotavirus strains.
Clin Diagn Lab Immunol. 1998;5:527-30.
9. Glass RI, Bhan MK, Ray P, Bahl R, Parashar UD,
Greenberg H, et al. Development of candidate rotavirus vaccines
derived from neonatal strains in India. J Infect Dis. 2005 ;192(Suppl
1):S30-5.
10. Ramani S, Kang G. Burden of disease and molecular
epidemiology of group A rotavirus infections in India. Indian J Med Res.
2007;125:619-32.
11. Kelkar SD, Bhide VS, Ranshing SS, Bedekar SS.
Rapid ELISA for the diagnosis of rotavirus. Indian J Med Res.
2004;119:60-5.
12. Kang G, Desai R, Arora R, Chitamabar S, Naik TN,
Krishnan T, et al. Diversity of circulating rotavirus strains in
children hospitalized with diarrhea in India, 2005-2009. Vaccine.
2013;31:2879-83.
13. Banerjee I, Gladstone BP, Le Fevre AM, Ramani S,
Iturriza-Gomara M, Gray JJ, et al. Neonatal infection with
G10P[11] rotavirus did not confer protection against subsequent
rotavirus infection in a community cohort in Vellore, South India. J
Infect Dis. 2007;195:625-32.
14. Rajasekaran D, Sastri NP, Marathahalli JR, Indi
SS, Pamidimukkala K, Suguna K, et al. The flexible C terminus of
the rotavirus non-structural protein NSP4 is an important determinant of
its biological properties. J Gen Virol. 2008;89:1485-96.
15. Bhowmick R, Halder UC, Chattopadhyay S, Nayak MK,
Chawla-Sarkar M. Rotavirus-encoded nonstructural protein 1 modulates
cellular apoptotic machinery by targeting tumor suppressor protein p53.
J Virol. 2013;87:6840-50.
16. Gladstone BP, Ramani S, Mukhopadhya I, Muliyil J,
Sarkar R, Rehman AM, et al. Protective effect of natural
rotavirus infection in an Indian birth cohort. N Engl J Med.
2011;365:337-46.
17. Sarkar R, Gladstone BP, Warier JP, Sharma SL,
Raman U, Muliyil J, et al. Rotavirus and other diarrheal disease
in a birth cohort from Southern Indian community. Indian Pediatr.
2016:53:583-8.
18. Lopman BA, Pitzer VE, Sarkar R, Gladstone B,
Patel M, Glasser J, et al. Understanding reduced rotavirus
vaccine efficacy in low socio-economic settings. PLoS One.
2012;7:e41720.
19. Narang A, Bose A, Pandit AN, Dutta P, Kang G,
Bhattacharya SK, et al. Immunogenicity, reactogenicity and safety
of human rotavirus vaccine (RIX4414) in Indian infants. Hum Vaccin.
2009;5:414-9.
20. Lokeshwar MR, Bhave S, Gupta A, Goyal VK, Walia
A. Immunogenicity and safety of the pentavalent human-bovine (WC3)
reassortant rotavirus vaccine (PRV) in Indian infants. H um Vaccin
Immunother. 2013;9:172-6.
21. Vashishtha VM, Choudhury P, Kalra A, Bose A,
Thacker N, Yewale VN, et al. Indian Academy of Pediatrics. Indian
Academy of Pediatrics (IAP) recommended immunization schedule for
children aged 0 through 18 years—India, 2014 and updates on
immunization. Indian Pediatr. 2014;51:785-800.
22. Bhandari N, Rongsen-Chandola T, Bavdekar A, John
J, Antony K, Taneja S, et al. India Rotavirus Vaccine Group.
Efficacy of a monovalent human-bovine (116E) rotavirus vaccine in Indian
infants: A randomised, double-blind, placebo-controlled trial. Lancet.
2014;383:2136-43.
23. Megiddo I, Colson AR, Nandi A, Chatterjee S,
Prinja S, Khera A, et al. Analysis of the Universal Immunization
Programme and introduction of a rotavirus vaccine in India with IndiaSim.
Vaccine. 2014;32 Suppl 1:A151-61.
24. Esposito DH, Tate JE, Kang G, Parashar UD.
Projected impact and cost-effectiveness of a rotavirus vaccination
program in India, 2008. Clin Infect Dis. 2011;52:171-7.
25. Arora R, Swaminathan S. Ready to measure impact?
The introduction of rotavirus vaccine in India. Indian Pediatr.
2016;53:565-7.
26. Mathew A, Srinivasan R, Venugopal S, Kang G.
Direct medical costs in children with rotavirus and non-rotavirus
diarrhea admitted to a pediatric intensive care unit and high dependency
unit in Delhi. Indian Pediatr. 2016;53:639-41.
27. Jacob J, Joseph TK, Srinivasan R, Kompithra RZ,
Simon A, Kang G. Direct and indirect costs of pediatric gastroenteritis
in Vellore, India. Indian Pediatr. 2016;642-4.
28. Rao TS, Arora R, Khera A, Tate JE, Parashar U,
Kang G; Indian Rotavirus Vaccine Working Group. Insights from global
data for use of rotavirus vaccines in India. Vaccine. 2014;32(Suppl
1):A171-8.
29. Mathew MA, Venugopal S, Arora R, Kang G.
Leveraging the National Rotavirus Surveillance Network for monitoring
intussusception. Indian Pediatr. 2016;53:635-8.
30. Mehendale S, Venkatasubramanian S, Girish Kumar
CP, Kang G, Gupte MD, Arora R. Expanded Indian National Rotavirus
Surveillance Network in the context of rotavirus vaccine introduction.
Indian Pediatr. 2016;53:575-81.
31. Girish Kumar CP, Venkatasubramanian S, Kang G,
Arora R, Mehendale S, for the National Rotavirus Surveillance Network.
Profile and trends of rotavirus gastroenteritis in under-five children
in India (2012-2014): Preliminary report of the Indian National
Rotavirus Surveillance Network. Indian Pediatr. 2016;53:619-22.
32. Kumar A, Basu S, Vashishtha VM, Choudhury P.
Burden of rotavirus diarrhea in under-five Indian children. Indian
Pediatr. 2016;53:607-17.
33. Teotia N, Upadhyay A, Agarwal S, Garg A, Shah D.
Rotavirus diarrhea in children presenting to an urban hospital in
western Uttar Pradesh, India. Indian Pediatr. 2016;53:627-9.
34. Maher G, Pradhan G, Shetty S, Ranshing S, Dample
A, Chitambar S. Rotavirus infection in children with acute
gastroenteritis in Aurangabad, central Maharashtra. Indian Pediatr.
2016;53:631-3.
35. Gupta M, Singh MP, Guglani V, Mahajan KS.
Hospital-based surveillance of rotavirus diarrhea among under-five
children in Chandigarh. Indian Pediatr. 2016;53:651-2.
36. Paul A, Babji S, Sarkar R, Lazarus RP, Kang G.
Rotavirus-specific salivary and fecal IgA in Indian children and adults.
Indian Pediatr. 2016;53:601-6.
37. Premkumar P, Lopman B, Ramani S, Paul A,
Gladstone B, Muliyil J, et al. Association of serum antibodies
with protection against rotavirus infection and disease in South Indian
children. Vaccine 2014;32(Suppl 1);A55-61.
38. Rongsen-Chandola T, Strand TA, Goyal N, Flem E,
Rathore SS, Arya A, et al. Effect of withholding breastfeeding on
the immune response to a live oral rotavirus vaccine in North Indian
infants. Vaccine. 2014;32(Suppl 1):A134-9.
39. Kompithra RZ, Paul A, Manoharan D, Babji S,
Sarkar R, Mathew LG, et al. Immunogenicity of a 3 dose and 5 dose
oral human rotavirus vaccine (RIX4414) schedule in south Indian infants.
Vaccine. 2014;32;(Suppl 1):A129-33.

