|
|
|
Indian Pediatr 2015;52:
613-615 |
 |
Sevelamer Hydrochloride for Tumor Lysis
Syndrome-related Hyperphosphatemia
|
|
Harsha Prasada L
From Department of Pediatrics, Kasturba Medical
College Hospital, Mangalore (Manipal University), Karnataka, India.
Correspondence to: Dr Harsha Prasada L,
Department of Pediatrics, Kasturba Medical College Hospital, Mangalore
575 001, Karnataka.
Email: [email protected]
Received: December 02, 2014.
Initial review: January 27, 2015.
Accepted: May 30, 2015.
|
|
Background: Tumour lysis syndrome
is associated with high levels of uric acid, phosphate and potassium
along with low levels of calcium and abnormal renal function. Sevelamer,
an oral phosphate-binder is used in the treatment of hyperphosphatemia
in children and adults on hemodialysis. Case characteristics: Two
children with T-cell acute lymphoblastic leukemia who presented with a
high tumour load and developed tumour lysis syndrome. Observation:
Both children received Rasburicase and Sevelamer hydrochloride. The
serum phosphate reduced to normal levels within 24-48 hrs of initiation
of sevelamer hydrochloride. Message: Sevelamer appears to be an
effective treatment for hyperphosphatemia associated with tumour lysis
syndrome.
Keywords: Acute lymphoblastic leukemia,
Hyperphosphatemia, Sevelamer hydrochloride, Tumour lysis syndrome.
|
|
A
cute lymphoblastic leukaemia (ALL) is the
commonest type of cancer seen among children contributing to almost one
fourth of all childhood cancers [1]. In developing countries, these
children present late when the disease has progressed to an advanced
stage with a high tumour load [2], and hence have a high risk of
developing a tumor lysis syndrome. The standard treatment of hyper-leukocytosis
associated with tumor lysis syndrome is hyperhydration and allopurinol
along with Rasburicase (Urate oxidase) to control very high uric acid
levels. If in spite of hyper-hydration the serum phosphate levels remain
high, it is then treated with aluminium hydroxide [3]. Sevelamer is a
non-calcium phosphate binder which is not absorbed from the
gastrointestinal tract, and has proven efficacy in reducing the serum
phosphate levels in chronic kidney disease and in patients on
hemodialysis [4,5]. We report two children with tumor lysis syndrome
where we used Sevelamer hydrochloride to treat hyperphosphatemia.
Case Report
Case 1: A seven-year-old boy reported with a
10 day history of progressive fever, associated with facial swelling and
bruises for 2 days. On examination he had bulky lymph nodes in the neck
associated with hepatosplenomegaly. His chest X-ray was
suggestive of superior mediastinal lymphadenopathy. His initial blood
results showed the following: Hb 9.3 g/dL, total leukocyte count 51×10 9/L,
and platelet count 7×109/L.
The blood film was suggestive of acute leukemia and was later confirmed
to be T-cell ALL. His other blood tests revealed: uric acid 18
mg/dL, phosphate 8 mg/dL, potassium 4.6 mmol/L, calcium 8.6 mg/dL and
LDH 1932 units. He was started on hyper-hydration (3L/m2)
by maintaining a urine output above 3mL/kg/hr using regular doses of
furosemide. Following a dose of rasburicase (0.2 mg/kg), the uric acid
levels came down to within the normal range (Fig. 1). The
serum creatinine was initially 1.1 mg/dL and reached a maximum of 1.9
mg/dL. The serum phosphate level started to rise on the day 2 of
admission to a maximum of 12 mg/dL, along with signs of hypocalcaemic
tetany (Serum calcium 5.7 mg/dL), which was treated with calcium
gluconate injections on two occasions. In addition to increasing fluids
to a maximum 5 L/m2,
sevelamer hydrochloride 400 mg was given orally every 8 hrs, following
which the phosphate levels were controlled in 48 hrs (Fig.1).
The child was started on UKALL 2003 protocol and is currently 6 months
from the date of diagnosis.
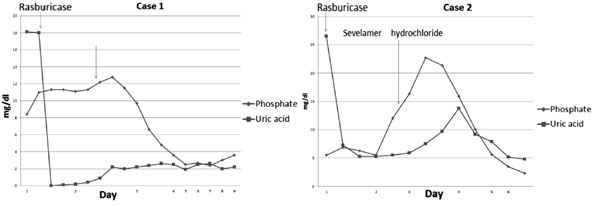 |
|
Fig.1 Changes in phosphate and uric
acid and sesporise to Sevelamer hydrochloride in the two
children.
|
Case 2: A 10-year-old boy presented with
fever for 2 weeks associated with skin rashes, and facial swelling for 5
days. He had generalized lymphadenopathy with large cervical, parotid
gland swelling and hepatosplenomegaly. The chest X-ray showed
mediastinal widening. His initial blood results showed the following: Hb
3g/dL, total leukocyte count 395 ×109/L
cells/cc, and platelets count of 40×109/L.
The initial uric acid was 26.5 mg/dL (Fig. 1), LDH 5674
units, potassium 6.4 mmol/l, calcium 9 mg/dL, phosphate 5.5 mg/dL and
the serum creatinine was 2.0 mg/dL. He was started on intravenous fluids
at 3 L/m2/day. A single dose
of rasburicase (3mg) controlled the hyperuricemia within 2 hours.
However, serum phosphate and creatinine level started to rise by day-2,
to 22 mg/dL and 2.4 mg/dL, respectively. Intravenous fluids were
increased to 5 L/m2/d and
urine output was maintained throughout with regular furosemide doses. He
also developed hypocalcemic tetany which was treated with calcium
gluconate injection. Oral Sevelamer hydrochloride 400 mg, every 8 hrs,
was given following which his serum phosphate levels came to within
normal levels in 48 hours. The flow cytometry result confirmed T cell
Acute Lymphoblastic Leukemia and the child was subsequently started on
chemotherapy as per the UKALL 2003 protocol.
Discussion
Hyperphosphatemia in children is usually defined when
serum phosphate level is above 6.6 mg/dL (2.1 mmol/L). In malignancies,
hyperphosphatemia occurs due to release of phosphate from the malignant
cells during degradation. Hyperphosphatemia can cause nausea, vomiting,
seizures, hypocalcemia, metastatic calcifi-cation, nephrocalcinosis and
acute renal failure. In some cases of childhood cancers, phosphate level
could go up in spite of aggressive hydration therapy, and may require
hemodialysis. Aggressive hydration and aggressive diuresis will treat
most cases of tumour lysis syndrome provided the kidney function is
within normal limits [6].
Oral phosphate binders such as aluminium hydroxide
can reduce serum phosphate level, but have adverse effects such as
diarrhea. There are mainly three types of non-calcium-based phosphate
binders available: Sevelamer, Lanthanum carbonate, and magnesium salts.
Sevelamer is the only non-calcium-containing phosphate binder that does
not have potential for systemic accumulation [6]. It is well tolerated
except for an increased incidence of metabolic acidosis [7]. Other side
effects include abdominal pain, diarrhea, nausea-vomiting, muscle
cramps, hypocalcemia, headache and hypermagnesemia [8]. In children with
end stage renal disease, it has been used in doses of 100 mg/kg/day to
130 mg/kg/day, every 8 hours [7,9].
In an earlier report [10], 13 children received
sevelamer at a mean dose of 400 mg twice a day for treatment of tumor
lysis syndrome related hyper-phosphatemia. Significant reduction in
serum phosphate was seen in 11 children. In both of our cases, serum
phosphate levels were high enough to initiate hemodialysis; however,
performing haemodialysis in newly diagnosed ALL is challenging in view
of low platelet counts. Use of Sevelamer hydrochloride avoided
hemodialysis and reduced the serum phosphate levels within 48 hours. We
conclude that Sevelamer hydrochloride can be used for short duration to
reduce the high phosphate levels associated with tumor lysis syndrome.
Funding: None; Competing interests: None
stated.
References
1. Satyanarayana L, Asthana S, Labani SP. Childhood
cancer incidence in India: A review of population-based cancer
registries. Indian Pediatr. 2014:51:218-20.
2. Advani S, Pai S, Venzon D, Adde M, Kurkure PK,
Nair CN, et al. Acute lymphoblastic leukemia in India: An
analysis of prognostic factors using a single treatment regimen. Ann
Oncol. 1999:10:167-76.;
3. Rajendran A, Bansal D, Marwaha RK, Singhi. Tumor
lysis syndrome. Indian J Pediatr 2013:80:50-4.
4. Chertow GM, Burke SK, Lazarus JM, Stenzel KH,
Wanbolt D, Goldberg D, et al. Poly [allylanmine hydrochloride] (Renagel):
A noncalcemic phosphate binder for the treatment of hyperphosphatemia in
chronic renal failure. Am J Kidney Dis. 1997;29:66-71.
5. Mahdavi H, Kuizon, BD, Gales B, Wang HJ, Elashoff
RM, Salusky IB. Sevelamer hydrochloride: An effective phosphate binder
in dialyzed children. Pediatr Nephrol. 2003:18:1260-4.
6. Malberti F. Hyperphosphataemia: Treatment options.
Drugs. 2013:73:673-88.
7. Pieper AK, Haffner D, Hoppe B, Dittrich K, Offner
G., Bonzel K, et al. A randomized crossover trial comparing
sevelamer with calcium acetate in children with CKD. Am J Kidney Dis.
2006:47:625-35.
8. Mitsopoulos E, Griveas I, Zanos S, Anagnostopoulos
K, Giannakou A, Pavlitou A, et al. Increase in serum magnesium
level in haemodialysis patients receiving sevelamer hydrochloride. Int
Urol Nephrol. 2005:37:321-8.
9. Storms LE, Chicella MF, Dice JE. Sevelamer therapy
for pediatric end-stage renal disease. Pharmacotherapy. 2006:26:410-3
10. Abdullah S, Diezi M, Sung L, Dupuis LL, Geary D,
Abla O. Sevelamer hydrochloride: A novel treatment of hyperphosphatemia
associated with tumor lysis syndrome in children. Pediatr Blood Cancer.
2008:51:59-61.
|
|
|
 |
|

