This randomized controlled trial was done to assess
whether endotracheal suctioning of nonvigorous infants born through
meconium stained amniotic fluid (MSAF) reduces the risk and
complications of meconium aspiration syndrome (MAS). Term, nonvigorous
babies born through MSAF were randomized to endotracheal suction or no
suction groups (n=61 in each). Risks of MAS, complications of MAS and
endotracheal suction, mortality, duration of neonatal intensive care
unit stay, and neurodevelopmental outcome at 9 months were assessed. In
total, 39 (32%) neonates developed MAS and 18 (14.8%) of them died.
There were no significant differences in MAS, its severity and
complications, mortality, and neurodevelopmental outcome for the two
groups. One infant had a complication of endotracheal suctioning, which
was mild and transient. The authors conclude that current practice of
routine endotracheal suctioning for nonvigorous neonates born through
MSAF should be further evaluated.
Commentary
Relevance: Fetal passage of meconium in utero
is a worrisome event because of the risk of meconium aspiration syndrome
(MAS), which carries threat of mortality to the extent of 5-40% [1]. In
addition, there are several unpleasant sequelae affecting the
respiratory system, and neuro-development in later life. Some of the
dangerous respiratory consequences of MAS are related to airway
obstruction and air-leak. However, there are also chemical effects
mediated by inflammation and inactivation of surfactant. Formerly, the
standard of care was nasopharyngeal and oropharyngeal suction of the
infant’s airway even before delivery. However, the evidence of benefit
from this intervention was not demonstrated in a meta-analysis of 4
trials [2], and this has now been abandoned altogether in active
vigorous babies. In contrast, current guidelines still advocate
inspection of the airway and endotracheal suctioning in
depressed/non-vigorous babies [3,4], probably because of absence of
evidence to change practice in this group of vulnerable neonates. The
general practice in such babies is to look for particulate meconium and
undertake endotracheal suction if it is present [5]. However, recent
reports suggest that this may not significantly reduce the risk of MAS
[6]. There are emerging views that non-vigorous babies may also not
require endotracheal suction. Against this backdrop, the recent trial by
Chettri, et al. [7] is a valuable addition to literature. The
trial [1] details are summarized in Table I.
|
TABLE I Summary of the Trial Details
|
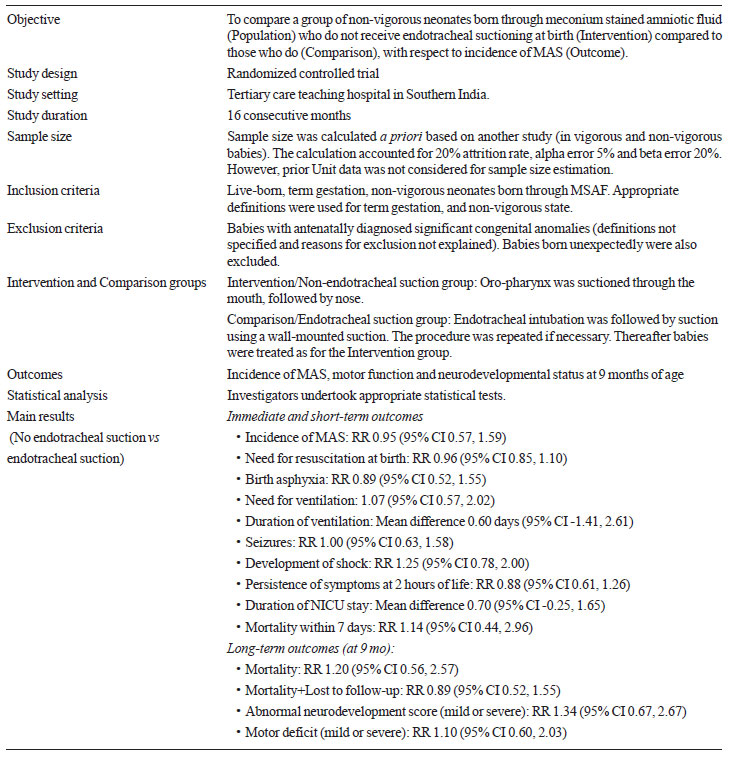 |
Critical appraisal: The RCT was planned
and executed well. Table II summarizes the methodological
characteristics. Overall, the trial qualifies for medium risk-of-bias
status. There are several refinements that make this trial noteworthy.
First, precise definitions have been used; and where relevant,
components of definitions (of various clinically used terms) have also
been explicitly clarified and defined. Further, the primary outcome
(incidence of MAS) has been supplemented with data on a variety of
clinically important parameters that are both patient-centric as well as
relevant to the managing team. The instruments used to evaluate
long-term outcomes were designed for Indian infants, and hence are
likely to have reliability and replicability in Indian settings. The
investigators have drawn conservative conclusions from their findings,
suggesting that this trial demands further evaluation of the time-honored
practice, rather than immediate change in practice. This is pertinent
because data from 122 babies may be insufficient to identify any
subgroups of non-vigorous neonates that may benefit (or alternatively be
harmed) from endotracheal suction.
|
Table II Methodological Appraisal of The
Trial
|
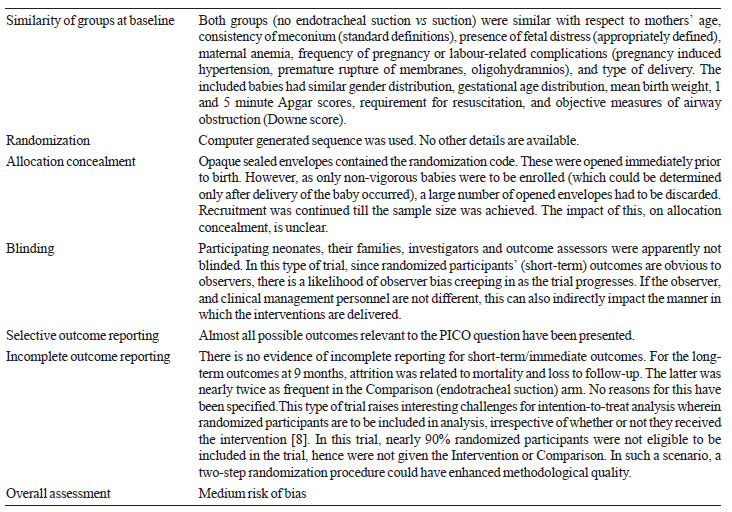 |
Extendibility: The RCT was conducted in a
teaching hospital in India itself, making it easier to replicate the
procedures followed in the trial, and extend the results to other
similar institutions in the country and region. The trial site is a
tertiary care institution, and hence better equipped in terms of
manpower and resources, to deal with exigencies that arise. This may be
particularly important because proper endotracheal suction itself needs
considerable training, and can be associated with complications [9]. For
this reason, the results of the trial may not be similar in other units
caring for newborn babies.
Another issue is that the trial found similar
outcomes in babies not receiving endotracheal suction and in those
receiving suction; however neither was superior (or inferior) for any
outcome parameter. This suggests that neonatology units and specialists
can consider change in practice only after carefully collating existing
local data (for a reasonable period of time), so that the impact of
change (if any) can be documented and interpreted correctly. On the
research front, the data from this trial would contribute to a
systematic review and meta-analysis of similar trials (as and when they
are reported).
Conclusions: This well conducted randomized trial
shows that in babies born through meconium stained amniotic fluid, who
are non-vigorous at birth, omission of the practice of endotracheal
suction, yields comparable short-term and long-term outcomes to those
who receive endotracheal suction.
Joseph L Mathew
Department of Pediatrics,
PGIMER, Chandigarh, India.
Email:
[email protected]
References
1. Yurdakök M. Meconium aspiration syndrome: do we
know? Turk J Pediatr. 2011;53:121-9.
2. Halliday HL. Endotracheal intubation at birth for
preventing morbidity and mortality in vigorous, meconium-stained infants
born at term. Cochrane Database Syst Rev. 2001;1:CD000500.
3. Roehr CC, Hansmann G, Hoehn T, Bührer C. The 2010
Guidelines on Neonatal Resuscitation (AHA, ERC, ILCOR): similarities and
differences – what progress has been made since 2005? Klin Pediatr.
2011;223:299-307.
4. Kattwinkel J, Perlman JM, Aziz K, Colby C,
Fairchild K, Gallagher J, et al. Part 15: neonatal resuscitation:
2010 American Heart Association Guidelines for Cardiopulmonary
Resuscitation and Emergency Cardiovascular Care. Circulation.
2010;122:S909 –19.
5. Green DA. Adaption of suction connectors for use
in meconium aspiration syndrome. Trop Doct. 2010;40:33-7.
6. Michel F, Nicaise C, Camus T, Di-Marco JN,
Thomachot L, Vialet R, et al. Management of newborns with
meconium-stained amniotic fluid: prospective evaluation of practice. Ann
Fr Anesth Reanim. 2010;29:605-9.
7. Chettri S, Adhisivam B, Bhat BV. Endotracheal
suction for nonvigorous neonates born through meconium stained amniotic
fluid: A randomized controlled trial. J Pediatr. 2015;166:1208-13.
8. No authors listed. Rationale for Concern About
Bias. Available from:
http://handbook.cochrane.org/chapter_8/8_13_1_rationale_for_concern_about_bias.htm.
Accessed June 13, 2015.
9. Velaphi S, Vidyasagar D. The pros and cons of suctioning at the
perineum (intrapartum) and post-delivery with and without meconium.
Semin Fetal Neonatal Med. 2008; 13: 375-382.

