|
|
|
Indian Pediatr 2014;51: 571-574 |
 |
Antimicrobial Prophylaxis for Children with
Vesicoureteral Reflux
Source Citation: The RIVUR Trial
Investigators. N Engl J Med. 2014;doi: 10.1056/NEJMoa1401811
|
|
|
|
Summary
This is a multi-centric randomized controlled trial
[1] comparing cotrimoxazole prophylaxis versus no prophylaxis in
children (<6 years old) with vesico-ureteral reflux (VUR) detected after
a first or second episode of urinary tract infection (UTI). The trial
was designed to determine efficacy in preventing recurrence of UTI and
renal scarring, as well as antibiotic resistance patterns. It was
conducted across 19 sites in the United States, recruiting subjects over
nearly five years, with follow-up for at least two years. Among 86%
children who completed the study, the investigators observed lower
recurrence of UTI in the intervention group (14.8%) in comparison to
controls (27.4%). Intention-to-treat analysis showed similar results but
lesser magnitude of difference (25.5% vs 37.4%). However, there
was no difference in the incidence of renal scarring (new scarring or
worsening of pre-existing scarring); 11.9% and 10.2% in the intervention
and control groups, respectively. The prevalence of antibiotic
resistance increased significantly with prophylaxis. The investigators
concluded that antibiotic prophylaxis is useful to prevent repeat
episodes of UTI in children with VUR.
Commentaries
Pediatric Nephrologist’s Viewpoint
The RIVUR trial conducted in North America randomized
607 children aged below 6 years, with VUR grades I-IV, after a UTI, to
receive either trimethoprim–sulfamethoxazole or placebo for 2 years [1].
Thirty-nine of 302 children on prophylaxis had UTI as compared to72 of
305 children on placebo [relative risk 0.55 (95% CI, 0.38 to 0.78)].
Benefits were more in children with febrile index UTI and in those with
bladder-bowel dysfunction (BBD). There was no difference in renal
scarring between the two groups. More children on prophylaxis (63%) than
on placebo (19%) had resistant isolates.
A very small number of boys (9%) were enrolled,
limiting applicability of its results to boys. While primary VUR is
commonly reported in girls, such huge gender disparity has not been
shown outside US [2-4]. This is important as boys did not benefit from
prophylaxis in the Swedish study [4]. Results of subgroup analyses (with
reasonable number of subjects and event rates) show that effect of
prophylaxis was not significant in grade III-IV VUR and in absence of
BBD. Thus it seems that prophylaxis is beneficial to a distinct patient
population comprising of girls with low grade reflux and BBD.
Increasingly, VUR is recognized as a heterogeneous condition with
regional and genetic differences [5]. It appears from this trial that
prophylaxis would reduce morbidity related to UTI but not long-term
consequences of renal scarring (hypertension, renal failure) at the cost
of increased antimicrobial resistance. A prudent way forward will be
that the use of prophylaxis is based on risk-stratification rather than
mere presence of reflux.
Pankaj Hari
Department of Pediatrics, AIIMS, New Delhi, India.
[email protected]
Pediatric
Surgeon’s Viewpoint
The present study proves the supremacy of
antimicrobial prophylaxis over watchful waiting approach for patients
with grade I to grade IV VUR. Of the grades of VUR studied, the
pediatric surgeons and pediatric urologists are usually involved in the
management of children with grade IV VUR. For lower grades of VUR, we
are consulted only when there are repeated episodes of breakthrough
infections, or when there are issues about non-compliance. One of the
shortfalls of this study is to study grades I to IV VUR together. The
authors should have studied patients with grades I and II, and those
with grades III and IV reflux, separately. Second, there is no mention
whether those patients with bladder and bowel dysfunction had any
urodynamic studies, or had concomitant bladder and bowel dysfunction
management. For the patients with grade III and IV reflux, surgical
correction and endoscopic management of VUR should also have been added.
An earlier study [6] – also referred by the authors of this paper –
documented that the incidence of pyelonephritis was significantly higher
in the medical group than the surgical group [6]. Endoscopic management
is known has equivocal results in comparison to surgical correction in
grades III and IV VUR [7]. So, the management of VUR should entail as
follows: Grade I and II – antimicrobial prophylaxis or watchful waiting,
Grade III and IV – endoscopic managemen,t and Grade V – surgical
correction. RIVUR trial data is relevant mainly for group I and II VUR.
Yogesh Kumar Sarin
Department of Pediatric Surgery, MAMC,
New Delhi, India.
Email: [email protected]
Evidence-based-medicine Viewpoint
Relevance: It is reported that UTI occurs
in 8-10% girls and 2-3% boys during infancy and childhood [8,9], with 5%
risk of recurrent infections and renal scarring [10] that may be
associated with long-term complications, including hypertension and
chronic renal disease [11]. Children with VUR are at increased risk of
recurrent infections and more serious consequences [12]. Therefore,
recent national [13] and international [14] guidelines recommend
screening for VUR and antibiotic prophylaxis after the first confirmed
episode of UTI. However, a previous exploration of evidence [15], did
not demonstrate reduction of recurrence of UTI with antibiotic
prophylaxis, in children with or without VUR. Two systematic reviews
[16,17] also did not find strong evidence of benefit in either group of
children. Against this backdrop, this RCT [1] is both relevant and
timely.
Critical appraisal: This study is an
example of a well-designed and well-conducted randomized trial. There
was appropriate allocation sequence generation and blinding. Multiple
outcomes were determined, with stringently defined UTI being the primary
outcome. No urine bags were used for specimen collection. Follow-up
nuclear scans for renal scarring were also read by pediatric nuclear
medicine experts. The sample size was calculated a priori, and
almost 86% children could be followed up for the primary outcome.
Follow-up duration was sufficiently long to record development of the
relevant outcomes. Data were analyzed as per protocol as well as by
intention-to-treat analysis. Although compliance to prophylaxis was less
than perfect, it probably matches the pattern in real-life. Overall, the
study is a methodologically high-quality trial, with low risk of bias.
Based on these characteristics, it should be
relatively easy to accept the reported findings, especially as it is in
line with other recent well-designed studies [18,19]. The difficulty
arises on two fronts: (i) the overall significance of the new data in
this trial, and (ii) its implications for practice.
The two Cochrane reviews [16,17] did not demonstrate
statistically significant reduction in risk of recurrence of UTI in
children with VUR, but these excluded a couple of large relevant trials
[4,19]. This necessitates a fresh meta-analysis pooling data from the
missed trial and the current RCT [1], which shows (Fig. 1)
that the relative risk of recurrence of UTI with prophylaxis is 0.70
(95% CI 0.48, 1.04; 7 trials, 1473 participants) when the
per-protocol data of new RCT are included. Re-analysis using ITT
data (Fig.2) shows almost similar results [RR 0.73
(95% CI 0.52, 1.03; 1553 participants). These data suggest that despite
this large well-designed study, the evidence is still equivocal and does
not demonstrate a statistically significant benefit of prophylaxis,
although there is a trend in this direction.
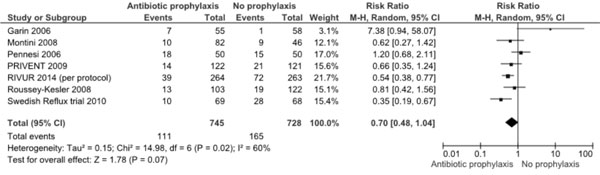 |
|
Fig. 1 Meta-analysis of antibiotic
prophylaxis versus no prophylaxis for prevention of UTI
recurrence (including RIVUR 2014 per protocol data).
|
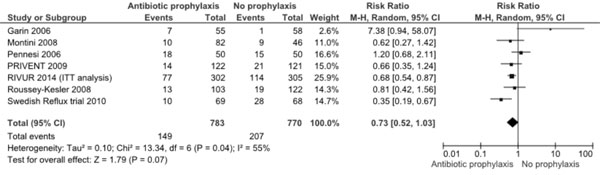 |
|
Fig 2: Meta-analysis of antibiotic
prophylaxis versus no prophylaxis for prevention of recurrence
of UTI (including RIVUR 2014 intention-to-treat analysis of
data).
|
Further, both Cochrane reviews and the current RCT
clearly showed that antibiotic prophylaxis did not prevent development
of renal scarring. Fresh meta-analysis (Fig. 3) including
the new data also confirms this (RR 0.62, 95% CI 0.27, 1.40; 6 trials,
1244 participants). This appears surprising because reduction in UTI
would be expected to reduce long-term scarring and its compli-cations.
Absence of this benefit calls for search of other more effective
approaches to manage VUR. In this context, recent experience with
dextranomer/hyaluronic acid polymer to treat VUR (20) is promising. In a
series with 54 children (81 VUR units), treatment resolved VUR in 72
(89%). Renal scarring remained status quo or regressed in 75%. If this
modality becomes routine, the management paradigm may shift from
watchful waiting with prophylaxis. Another important reason to lean away
from prophylaxis is the associated increase in prevalence of
antibiotic-resistant bacteria over time (>25% in this RCT), and episodes
of UTI caused by resistant bacteria (>65%). This suggests that
cotrimoxazole prophylaxis cannot be continued indefinitely. Fresh
research is required to study the pattern with antibiotic rotation
regimens.
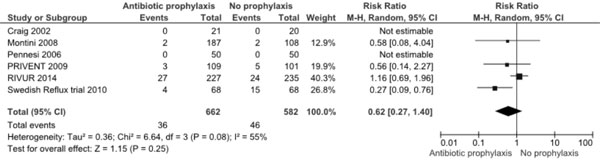 |
|
Fig. 3 Meta-analysis of antibiotic
prophylaxis versus no prophylaxis for prevention of new or
worsening renal scarring
|
Extendibility: There are several reasons
that the data from this study can be extended to the Indian setting. UTI
is fairly common, although appropriate diagnosis using stringent
criteria and appropriate urine collection methods, need improvement. E.
coli is the most commonly isolated organism in children [21], and
Cotrimoxazole the most commonly chosen agent for prophylaxis. On the
other hand, not all centers have access to modalities for detecting VUR
and/or renal scarring. In such settings, should physicians continue
prescribing prophylaxis (hoping for reduced recurrence of UTI but being
aware that it increases risk of episodes with resistant organisms, and
has no benefit on scarring) or abandon the practice altogether.
Unfortunately, current high quality evidence does not resolve the issue,
and may necessitate a well-designed RCT in Indian children.
Joseph L Mathew
Department of Pediatrics,
PGIMER, Chandigarh, India.
Email: [email protected]
References
1. The RIVUR Trial Investigators. Antimicrobial
prophylaxis for children with vesicoureteral reflux. N Engl J Med. 2014;
May 4. [Epub ahead of print]
2. Hannula A, Venhola M, Renko M, Pokka T, Huttunen
NP, Uhari M. Vesicoureteral reflux in children with suspected and proven
urinary tract infection. Pediatr Nephrol. 2010;25:1463-9.
3. Nakai H, Kakizaki H, Konda R, et al;
Prospective Study Committee of Reflux Nephropathy Forum, Japan. Clinical
characteristics of primary vesicoureteral reflux in infants: multicenter
retrospective study in Japan. J Urol. 2003; 169:309-12.
4. Brandström P, Esbjörner E, Herthelius M,
Swerkersson S, Jodal U, Hansson S. The Swedish reflux trial in children:
III. Urinary tract infection pattern. J Urol 2010;184:286-91
5. Williams G, Fletcher JT, Alexander SI, Craig JC.
Vesicoureteral reflux. J Am Soc Nephrol. 2008;19:847-62.
6. Weiss R, Duckett J, Spitzer A. Results of a
randomized clinical trial of medical versus surgical management of
infants and children with grades III and IV primary vesicoureteral
reflux (United States). The International Reflux Study in Children. J
Urol. 1992;148(5 Pt 2):1667-73.
7. Benoit RM, Peele PB, Docimo SG. The
cost-effectiveness of dextranomer/hyaluronic acid copolymer for the
management of vesicoureteral reflux: 1: substitution for surgical
management. J Urol. 2006;176:1588-92.
8. DeMuri GP, Wald ER. Imaging and antimicrobial
prophylaxis following the diagnosis of urinary tract infection in
children. Pediatr Infect Dis J. 2008;27:553-4.
9. Mishra OP, Abhinay A, Prasad R. Urinary infections
in children. Indian J Pediatr. 2013;80:838-43.
10. Coulthard MG, Lambert HJ, Keir MJ. Occurrence of
renal scars in children after their first referral for urinary tract
infection. BMJ. 1997;315 918-9.
11. Najar MS, Saldanha CL, Banday KA. .Approach to
urinary tract infections. Indian J Nephrol. 2009;19:129-39.
12. Hoberman A, Charron M, Hickey RW, Baskin M,
Kearney DH, Wald ER. Imaging studies after a first febrile urinary
tract infection in young children. N Engl J Med. 2003;348:195-202
13. Indian Society of Pediatric Nephrology,
Vijayakumar M, Kanitkar M, Nammalwar BR, Bagga A. Revised statement on
management of urinary tract infections. Indian Pediatr. 2011;48:709-17.
14. Subcommittee on Urinary Tract Infection, Steering
Committee on Quality Improvement and Management, Roberts KB. Urinary
tract infection: Clinical practice guideline for the diagnosis and
management of the initial UTI in febrile infants and children 2 to 24
months. Pediatrics. 2011;128:595-610.
15. Mathew JL. Antibiotic prophylaxis following
urinary tract infection in children: A systematic review of randomized
controlled trials. Indian Pediatr. 2010;47:599-605.
16. Williams G, Craig JC. Long-term antibiotics for
preventing recurrent urinary tract infection in children. Cochrane
Database Syst Rev. 2011;3:CD001534.
17. Nagler EVT, Williams G, Hodson EM, Craig JC.
Interventions for primary vesicoureteric reflux. Cochrane Database Syst
Rev. 2011;6:CD001532.
18. Craig JC, Simpson JM, Williams GJ, Lowe A,
Reynolds G, McTaggart SJ, et al. Antibiotic prophylaxis and
recurrent urinary tract infection in children. New Engl J Med.
2009;361:1748-59.
19. Garin EH, Olavarria F, Garcia Nieto V, Valenciano
B, Campos A, Young L. Clinical significance of primary vesicoureteral
reflux and urinary antibioticprophylaxis after acute pyelonephritis: A
multicenter, randomized, controlled study. Pediatrics. 2006;117:626-32.
20. Garge S, Menon P, Rao KLN, Bhattacharya A, Abrar
L, Bawa M, et al. Vesicoureteral reflux: Endoscopic therapy and
impact on health related quality of life. J Indian Assoc Pediatr Surg.
2013;18:11-15.
21. Sharan R, Kumar D, Mukherjee B. Bacteriology and
antibiotic resistance pattern in community acquired urinary tract
infection. Indian Pediat. 2013;50:707-8.
|
|
|
 |
|

