|
|
|
Indian Pediatr 2014;51:
539-543 |
 |
INCLEN Diagnostic Tool
for Epilepsy (INDT-EPI) for Primary Care Physicians:
Development and Validation
|
|
Ramesh Konanki, Devendra Mishra, Sheffali Gulati,
Satinder Aneja, Vaishali Deshmukh,
Donald Silberberg, Jennifer M Pinto, Maureen Durkin, Ravindra M Pandey,
MKC Nair,
Narendra K Arora and INCLEN Study Group*
From the Inclen Trust International, New Delhi, India
Correspondence to: Dr Narendra K Arora, Executive
Director, The Inclen Trust, International, F1/5, Okhla Industrial Area,
Phase-1, New Delhi, India.
Email: nkarora@inclentrust.org
Received: April 03, 2013;
Initial review: May 08, 2013;
Accepted: February 14, 2014.
*INCLEN Study Group: Core Group:
Alok Thakkar, Arun Singh, Gautam Bir Singh, Manju Mehta, Manoja K Das,
Monica Juneja, Nandita Babu, Paul SS Russell, Poma Tudu, Praveen Suman,
Rajesh Sagar, Rohit Saxena, Savita Sapra, Sharmila Mukherjee, Sunanda K
Reddy, Tanuj Dada, Vinod Bhutani. Extended Group:
AK Niswade, Archisman Mohapatra, Arti Maria, Atul Prasad, BC Das,
Bhadresh Vyas, GVS Murthy, Gourie M Devi, Harikumaran Nair, JC Gupta, KK
Handa, Leena Sumaraj, Madhuri Kulkarni, Muneer Masoodi, Poonam Natrajan,
Rashmi Kumar, Rashna Dass, Rema Devi, Sandeep Bavdekar, Santosh Mohanty,
Saradha Suresh, Shobha Sharma, Sujatha S Thyagu, Sunil Karande, TD
Sharma, Vinod Aggarwal, Zia Chaudhuri.
|
Objective: To evaluate the diagnostic accuracy of
a new diagnostic instrument for epilepsy – INCLEN Diagnostic Tool for
Epilepsy (INDT-EPI) – with evaluation by expert pediatric neurologists.
Study design: Evaluation of diagnostic test.
Setting: Tertiary care pediatric referral centers
in India.
Methods: Children aged 2-9 years, enrolled by
systematic random sampling at pediatric neurology out-patient clinics of
three tertiary care centers were independently evaluated in a blinded
manner by primary care physicians trained to administer the test, and by
teams of two pediatric neurologists.
Outcomes: A 13-item questionnaire administered by
trained primary care physicians (candidate test) and comprehensive
subject evaluation by pediatric neurologists (gold standard).
Results: There were 240 children with epilepsy
and 274 without epilepsy. The candidate test for epilepsy had
sensitivity and specificity of 85.8% and 95.3%; positive and negative
predictive values of 94.0% and 88.5%; and positive and negative
likelihood ratios of 18.25 and 0.15, respectively.
Conclusion: The INDT-EPI has high validity to
identify children with epilepsy when used by primary care physicians.
Keywords: Childhood neuro-developmental disorders,
Resource-limited settings, Psychometric evaluations.
Keywords: Efficacy, Immunization, Neisseria meningitidis,
Protection, Vaccine.
|
|
Epilepsy contributes to significant morbidity with
reported prevalence of 2.4-5.6 per 1000 in India [1-3]. However, nearly
75% of these do not receive appropriate treatment [4], many due to a
lack of proper diagnosis. The situation is no different in other
developing countries [5-9]. The reported rate of misdiagnosis of
epilepsy among pediatricians ranges from 30-39% [10-13]. The diagnosis
of epilepsy is mostly based on clinical history supported by neuro-imaging
and electroencephalography. In the absence of an objective "gold
standard" diagnostic test, the decision of a team of experienced
pediatric neurologists with access to all investigations may be
considered as nearest to the "gold standard" for diagnosis of childhood
epilepsy [14].
As per 2003 estimates, the Indian populations of over
1 billion were being served by only about 500 neurologists [4,15] most
of whom serve in large cities. Similarly, most pediatricians are also
concentrated in urban areas while the majority of Indians still live in
the villages and small towns where such expertise is not available. The
most effective way to reduce the treatment gap of people with epilepsy
in developing countries is delivery of epilepsy services through primary
health care [16]. Hence, there is a need to for a diagnostic instrument
for use by primary care physicians to help them in identifying cases
with epilepsy as well as ruling out non-epileptic events. There are no
comprehensive, validated tools that can be administered by such
physicians for diagnosis of epilepsy. The INCLEN Diagnostic Tool for
Epilepsy (INDT-EPI) has been developed with the major aim of increasing
the access to care for seizure disorders of large segments of
populations residing in rural areas and small towns where specialists
care may not be available. The present study was conducted to evaluate
the psychometric properties of this new instrument for childhood
epilepsy as part of a nation-wide, multi-centre prevalence study for
common neuro-developmental disorders among children aged 2-9 years.
Methods
This diagnostic test evaluation study was conducted
on children attending the pediatric neurology outpatient clinics of
three public sector tertiary care pediatric referral centers [All India
institute of Medical Sciences (AIIMS), Lady Hardinge Medical College
(LHMC) and Maulana Azad Medical College (MAMC)] in New Delhi, India.
These centers receive referred cases for complex medical problems as
well as simple ailments seen at primary care level, mostly from National
Capital Region and nearby states. Children (2-9 years) of either gender
attending the pediatric neurology outpatient clinics were eligible for
inclusion in the study. Children who had poor general condition
requiring admission (e.g. respiratory distress requiring supplemental
oxygen, altered sensorium, peripheral circulatory collapse, suspected
sepsis and bleeding), and those who were not accompanied by a primary
caregiver were excluded from the study. At each study site, a team of
two pediatric neurologists with at least three years experience in the
diagnosis and management of epileptic children, one study coordinator
and one graduate (MBBS) physician participated in the study. Ethical
approval was obtained from IndiaCLEN Review Board and the Institutional
Review Board of all the study sites. The instrument development and data
collection were done from January 2008 to April 2010.
Diagnostic instruments
Gold standard: The diagnosis of epilepsy was
established at each site by consensus of the two pediatric neurologists
following detailed history and physical examination with access to
electroencephalogram, computed tomography and/or magnetic resonance
imaging of Brain, as indicated. The instrument included a summary
assess-ment (diagnosis): ‘epilepsy’, ‘epilepsy with other neuro-developmental
disorder (NDD)’, "NDDs other than epilepsy" and "No NDD/Epilepsy".
Candidate test: INDT-EPI which has been
developed on the standard definitions of seizures and epilepsy proposed
by the International League Against Epilepsy (ILAE) [17] through
consensus among multidisciplinary national and international team of
experts (49 national and 6 international).
Instrument Development
The INDT-EPI was developed as consensus clinical
criteria (CCC) for diagnosing epilepsy by the Technical Advisory Group
(TAG) consisting of pediatricians, developmental pediatricians, child
psychiatrists, pediatric neurologists, pediatric otorhinolaryngology,
community physicians, clinical psychologists, special educators,
specialist nurses, speech therapists, occupational therapists, and
social scientists through a series of discussions and meetings using
Delphi method and over three rounds of 2-day workshops.
INDT-EPI included questions in simple language to
elicit the history of common seizure types (generalized and partial
motor seizures, absence seizures and myoclonic seizures) (questions
1,2,10,11), the number of seizures and duration between first and last
seizures is captured through question 3 and 4, provoked seizures such as
febrile seizures, seizures occurring during neuro-infections, with head
trauma or during systemic illnesses (question 5 for febrile seizures, 6
for acute symptomatic seizures, 7 for neonatal seizures) and seizure
mimics such as breath holding spells (question 8) and syncopal attacks
(question 9). Question 12 and 13 are final diagnosis. The instrument was
translated from English to Hindi and back translated to English before
the study was undertaken. The Hindi instrument was pretested in 20
children to look for difficulties in administering/understanding the
questions and time needed to complete assessment. The instrument is
available as Web Appendix I.
Enrolment and assessments
Enrolment was done through systematic random
sampling. Two computer-generated random numbers were provided to the
study coordinator daily in a sealed envelope. The first number (between
1 and 9) determined the starting point, and the second random number
(between 5 and 15) determined the nth number (sample interval) to be
sampled starting from the first random number. Every nth child in the
age group of 2-9 years was assessed for eligibility and enrolled after
obtaining written, informed consent from the primary caregiver
until the final sample was achieved. If consent or inclusion criterion
was not achieved, (n+1)th child was enrolled. The day’s enrollment
stopped once 15 children were enrolled in above manner or OPD
registration was over, whichever happened first. Consecutive study
subjects were enrolled in the above manner until desired number of
children were identified based on gold standard diagnosis. Since the
subjects were recruited from pediatric neurology outpatients, stoppage
of recruitment was linked to achieving desired sample of children with
‘no epilepsy’ and ‘no NDD’.
At all three sites, subjects were first administered
the INDT-EPI (candidate test) by the primary care physician and later
evaluated by the expert team of pediatric neurologists (gold standard).
Administration of INDT-EPI took approximately 30-40 minutes.
These findings were filled in a predesigned instrument, enclosed in
separate sealed, opaque envelop bearing the subject’s unique
identification number and handed over to the coordinator. The sealed
envelopes of expert team (gold standard) were opened at the end of day
by the coordinator, who was not part of the assessment team to enlist
the number of cases of epilepsy, epilepsy with other neuro-developmental
disorders (NDDs), NDDs other than epilepsy, and group with no epilepsy
or NDDs, based on the gold standard assessments.
Sample size: Assuming sensitivity and specificity
of INDT-EPI to be 85% with relative precision of 10% at 95%
confidence level, sample size was calculated to be 68 in each category.
To account for drop-outs, it was decided to enrol at least 80 children
in each category (epilepsy, epilepsy with other NDD, NDDs other than
epilepsy, and group with no epilepsy or NDD).
Training and quality assurance: INDT-EPI training
manual for administration and caregiver’s response interpretation was
prepared. General physicians were trained (eight hours of didactic
manual-based teaching and instrument administration on five cases each)
by pediatric neurologists during a two-day comprehensive, hands-on,
structured workshop.
The team of pediatric neurologists (gold standard)
was blinded to the assessment of the physician (candidate test). The
study coordinator at the site assessed children attending the
out-patient clinic for eligibility and enrolled them after taking
written, informed consent from the primary caregiver, but did not take
part in any of the assessments.
Statistical analysis: The data were analyzed
using STATA version.10. The utility and psychometric properties of
INDT-EPI were calculated in comparison with the assessments by the team
of pediatric neurologists.
Results
Out of 531 children assessed for eligibility, 514
(341 boys) were included; 11 children refused consent and 6 were not
accompanied by primary caregiver. Mean (SD) age of included children was
60.1 (1.0) months. Of the 240 children with epilepsy, 97 (40%) had only
epilepsy, and 143 (60%) had epilepsy with NDDs according to gold
standard. Of 274 children without epilepsy, 194 (71%) had NDDs other
than epilepsy, and 80 (29%) had no NDDs. Fig. 1 details
the study flow. Out of 240 children with epilepsy, 203 (84%) had
generalized or focal motor seizures, 12 (5%) had absence seizures, and
16 (6.6%) had myoclonic. The team of neurologists could not assign a
clear classification to 9 children. Table I details
the performance of INDT-EPI instrument in comparison to the gold
standard. The possible reasons for the false diagnoses (4.7% false
positives and 14.2% false negatives) by the candidate test are
summarized in Web Table I and
Web Table II.
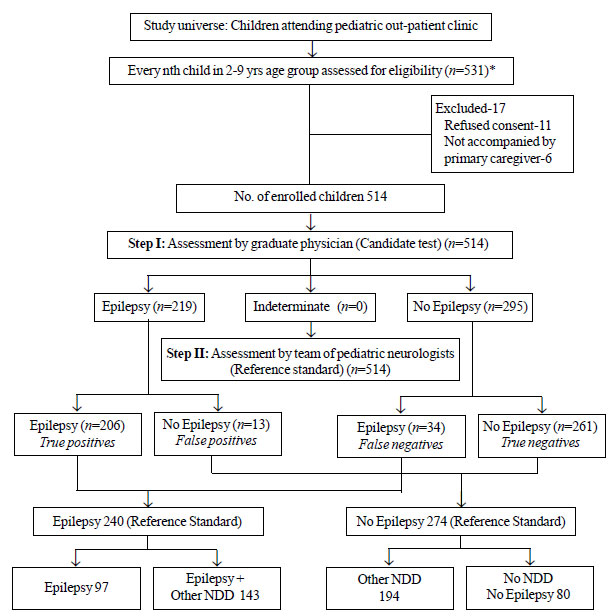 |
|
Fig. 1 The study plan.
|
TABLE I Psychometric Properties of INCLEN Diagnostic Tool for Epilepsy (INDT-EPI)
|
Sensitivity
|
85.8 (80.8-90.0) |
Specificity
|
95.3 (92.0-97.4) |
|
LR of positive test |
18.2 (10.6-30.8) |
LR of negative test |
0.15 (0.11-0.20)
|
|
Positive predictive value |
94.0 (90.6-96.8) |
Negative predictive value
|
88.5 (84.3-91.9) |
LR: Likelihood ratio; All values in %(95% CI).
|
Discussion
INDT-EPI for diagnosis of childhood epilepsy by the
primary care physician demonstrated good psychometric properties. To the
best of our knowledge, there are no validated instruments for diagnosing
epilepsy. The tools currently available include screening
questionnaires, with confirmation done by specialists [1,18-24].
Differentiating children with epilepsy from those
with other NDDs like cerebral palsy and intellectual disability
(overlapping symptomatology) is often challenging. The specificity of
INDT-EPI increased to 97.4% when it was administered to children with
other NDDs. The specificity of the instrument in subgroup of children
without any NDD was lower (90%) compared to subgroup with other NDDs
(97.4%). It is possible that parents of normal children are less likely
to be forthcoming in terms of thoughtful responses than parents of
children with other NDDs. This can also be attributed to different
health seeking behavior of the parents.
In an earlier study assessing the nature of multiple
events (epileptic or non-epileptic), it was seen that 4.6% children with
non-epileptic events were initially misdiagnosed as having epilepsy
(false positive) and 5.6% children with epilepsy were initially
diagnosed as having ‘no epilepsy’ (false negative) [14]. The assessments
in that study were comprehensive including detailed evaluation by a
panel of pediatric neurologists supported by electroencephalography and
neuro-imaging, when required. In the present study, the assessments were
done by graduate physicians trained to administer the structured
clinical instrument. The rate of false positives in the current study is
comparable to the above-mentioned study and is much lower compared with
the reported rates of misdiagnosis of epilepsy [10,11,13]. To minimize
the misclassification of epilepsy as acute symptomatic seizures (neuro-infections,
head trauma, and systemic illness), clear-cut definitions with durations
can be introduced for defining the seizures occurring in ‘close’
temporal association with brain infections as highlighted by the recent
ILAE guidelines [17,25]. With the addition of duration cut-off to define
acute symptomatic seizures, the sensitivity of the INDT-EPI is likely to
increase.
Limitation of the present study was that the included
subjects from the tertiary care referral centers might not be
representative of the community. The 240 patients of epilepsy out of 514
children reflect the referral bias in pediatric neurology outpatients.
The instrument, in its present form, does not have the provision for
differentiating between active and prevalent cases. The primary care
physician has to suspect epilepsy in his/her setting before the tool is
administered; tool should pick up both prevalent as well as active cases
in such situation.
To conclude, INDT-EPI is a useful tool for diagnosis
of childhood epilepsy by non-expert medical pro-fessionals (with
adequate training) in different clinical settings, and for future
epidemiological studies. This instrument can also be used in day-to-day
clinical practice for diagnosing epilepsy by the primary health care
physicians thereby expanding the care for epilepsy patients and reducing
diagnosis management gap in resource-limited settings. Further studies
on the instrument are recommended to assess its performance in different
community and healthcare settings.
Contributors: All authors have contributed,
designed and approved the study. NKA will act as a guarantor for this
work.
Funding: Ministry of Social Justice and
Empowerment (National Trust), National Institute of Health (NIH-USA);
Fogarty International Center (FIH), Autism Speaks (USA); Competing
interests: None stated.
|
What is Already Known?
• The diagnosis of epilepsy in children
requires evaluation by experienced pediatricians or pediatric
neurologists along with supporting investigations like EEG and
neuro-imaging.
What This Study Adds?
• The INDT-EPI tool for diagnosing epilepsy
has good sensitivity and specificity when used by primary care
physicians with short training.
|
References
1. Mani KS, Rangan G, Srinivas HV, Kalyanasundaram S,
Narendran S, Reddy AK. The yelandur study: A community-based approach to
epilepsy in rural south India–epidemiological aspects. Seizure.
1998;7:281-8.
2. Koul R, Razdan S, Motta A. Prevalence and pattern
of epilepsy (Lath/Mirgi/Laran) in rural Kashmir, India. Epilepsia. 1988;
29:116-22.
3. Radhakrishnan K, Pandian JD, Santhoshkumar T,
Thomas SV, Deetha TD, Sarma PS, et al. Prevalence, knowledge,
attitude, and practice of epilepsy in Kerala, south India. Epilepsia.
2000; 41:1027-35.
4. Sridharan R, Murthy BN. Prevalence and pattern of
epilepsy in India. Epilepsia. 1999; 40:631-6.
5. Gu L, Liang B, Chen Q, Long J, Xie J, Wu G, et
al. Prevalence of epilepsy in the People’s Republic of China: a
systematic review. Epilepsy Res. 2013;105:195-205.
6. Malik MA, Akram RM, Tarar MA, Sultan A. Childhood
epilepsy. J Coll Physicians Surg Pak. 2011; 21:74-8.
7. Benamer HT, Grosset DG. A systematic review of the
epidemiology of epilepsy in Arab countries. Epilepsia. 2009; 50:2301-4.
8. Somoza MJ, Forlenza RH, Brussino M, Centurión E.
Epidemiological survey of epilepsy in the special school population in
the city of Buenos Aires. A comparison with mainstream schools.
Neuroepidemiology. 2009; 32:129-35.
9. Calisir N, Bora I, Irgil E, Boz M. Prevalence of
epilepsy in Bursa city center, an urban area of Turkey. Epilepsia. 2006;
47:1691-9.
10. Chadwick D, Smith D. The misdiagnosis of
epilepsy. BMJ. 2002; 324:495-6.
11. Chinthapalli RN. Who should take care of children
with epilepsy? BMJ. 2003; 327:1413.
12. Cockerell OC, Hart YM, Sander JW, Shorvon SD. The
cost of epilepsy in the United Kingdom: an estimation based on the
results of two population-based studies. Epilepsy Res. 1994; 18:249-60.
13. Uldall P, Alving J, Hansen LK, Kibaek M, Buchholt
J. The misdiagnosis of epilepsy in children admitted to a tertiary
epilepsy centre with paroxysmal events. Arch Dis Child 2006;91:219-21.
14. Stroink H, van Donselaar CA, Geerts AT, Peters
ACB, Brouwer OF, Arts WFM. The accuracy of the diagnosis of paroxysmal
events in children. Neurology. 2003; 60:979-82.
15. Krishnamoorthy ES, Satishchandra P, Sander JW.
Research in epilepsy: development priorities for developing nations.
Epilepsia. 2003; 44 Suppl 1:5-8.
16. Carpio A, Hauser WA. Epilepsy in the developing
world. Curr Neurol Neurosci Rep. 2009; 9:319-26.
17. ILAE Commission Report. The epidemiology of the
epilepsies: future directions. Epilepsia. 1997; 38:614-8.
18. Placencia M, Sander JW, Shorvon SD, Ellison RH,
Cascante SM. Validation of a screening questionnaire for the detection
of epileptic seizures in epidemiological studies. Br J Neurol. 1992;
115:783-94.
19. Bharucha NE, Bharucha EP, Bharucha AE, Bhise AV,
Schoenberg BS. Prevalence of epilepsy in the Parsi community of Bombay.
Epilepsia. 1988; 29:111-5.
20. Gourie-Devi M, Gururaj G, Satishchandra P,
Subbakrishna DK. Neuro-epidemiological pilot survey of an urban
population in a developing country. A study in Bangalore, south India.
Neuroepidemiology. 1996; 15:313-20.
21. Osuntokun BO, Adeuja AO, Nottidge VA, Bademosi O,
Olumide A, Ige O, et al. Prevalence of the epilepsies in Nigerian
Africans: a community-based study. Epilepsia. 1987; 28:272-9.
22. Anand K, Jain S, Paul E, Srivastava A, Sahariah
SA, Kapoor SK. Development of a validated clinical case definition of
generalized tonic-clonic seizures for use by community-based health care
providers. Epilepsia. 2005; 46:743-50.
23. Das SK, Biswas A, Roy T, Banerjee TK, Mukherjee
CS, Raut DK, et al. A random sample survey for prevalence of
major neurological disorders in Kolkata. Indian J Med Res. 2006;
124:163-72.
24. Banerjee TK, Ray BK, Das SK, Hazra A, Ghosal MK,
Chaudhuri A, et al. A longitudinal study of epilepsy in Kolkata,
India. Epilepsia. 2010; 51:2384-91.
25. Beghi E, Carpio A, Forsgren L, Hesdorffer DC,
Malmgren K, Sander JW, et al. Recommendation for a definition of
acute symptomatic seizure. Epilepsia. 2010; 51:671-5.
|
|
|
 |
|

