|
The Indian Academy of Pediatrics Committee on
Immunization (IAPCOI) met on 24th
and 25th December 2011 in
Mumbai. IAPCOI members and invitees who attended the meeting are listed
in Annexure 1. The aim of the meeting was to discuss and debate
recent developments in the field and to issue recommendations based on
them, and to revise IAP Immunization Timetable for the year 2012. This
document presents the consensus recommendations, which arrived out of
that meeting.
Process for Issuing Recommendations
The process involves review of recent published
literature including standard text books, vaccine trials,
recommendations of reputed international bodies like ACIP of CDC, World
Health Organization (WHO) etc, post-marketing surveillance reports from
industry, cost-effective analysis, etc. More reliance is given to
studies emanating from India, especially on disease epidemiology, and
vaccines’ immunogenicity, efficacy, and safety studies. If knowledge
gaps are present then expert opinion is sought to fill the gaps. The
existing national immunization schedule and government policies are also
taken in to account while drafting recommendations. The recommendations
of IAPCOI are primarily for pediatricians in office practice. In
addition, IAPCOI also submits its position on incorporation of various
new vaccines in the national immunization schedule.
I. Proceedings and recommendations
The IAPCOI has taken following key decisions:
1. Categorization of vaccines: IAPCOI has
abolished the earlier categorization of vaccines in four categories [1].
Now there will be only two categories: one, the vaccines recommended by
IAP for routine use; two, the vaccines to be used in special
circumstances only.
2. IAP immunization timetable: Since
immunization schedules ought to be dynamic—adaptable to ongoing
epidemiological changes and rapid developments in vaccine sciences, it
is unanimously resolved to revise immunization timetable every year
rather than every two years as has been practiced so far.
3. Revised process for issuing recommendations:
It is decided to develop a uniform approach to making
explicit the evidence base for IAPCOI recommendations. The committee
will adopt a new evidence-based methodology, e.g. GRADE (Grades
of Recommendation Assessment, Development and Evaluation, for issuing
not only the future recommendations but to apply to existing
recommendations also, especially on newer vaccines. A subcommittee is
also constituted that will devise a new model based entirely on evidence
to grade the available evidences and on its basis decide the strength of
recommendations in 2-3 different categories. The main focus will be on
scientific evidence and transparency so that the system can be
reproducible and can also be reviewed by other experts.
4. Position papers: It is also decided to
prepare position papers on important vaccines and vaccine preventable
diseases highlighting committee’s stand on various issues on the format
of WHO position papers. Hib diseases and vaccines have been chosen for
the inaugural papers.
II. AIMS AND OBJECTIVES
• To review and issue recommendations on the
recent contentious issues pertaining to rotavirus, Hib, and
pneumococcal conjugate vaccines.
• To revise IAP Immunization Timetable for the
year 2012.
III. SPECIFIC RECOMMENDATIONS
A. Rotavirus Vaccine
In the light of recent publications and developments,
the following issues are considered for discussion:
1. Burden of rotavirus disease in India
According to most recent global estimates, India
accounts roughly 22% of deaths (98 621 deaths) due to rotavirus out of
global estimates of 453 000 deaths [2]. Along with India, Democratic
Republic of the Congo, Ethiopia, Nigeria, and Pakistan account for more
than half of all deaths attributable to rotavirus infections globally
[2]. Most of Indian studies are hospital-based. However, according to
one review that collated data from 46 epidemiological studies conducted
between 1990-2005, rotavirus positivity rates varied greatly between
different settings - diarrhea hospitalizations (20%), neonatal
infections (35%), symptomatic and asymptomatic infections in the
community (15.1% and 6.3%, respectively) and nosocomial enteric
infections (22.5%) [3]. The incidence of rotavirus positivity amongst
hospitalized children varies from 6-45% (20.8%) [3]. According to the
Indian Rotavirus Strain Surveillance Network (IRSN), established with 4
laboratories and 10 hospitals in 7 different regions of India, rotavirus
was found in approximately 39% of 4243 enrolled patients from December
2005 through November 2007 with greatest incidence seen among children
aged 6-23 months [4].
2. Efficacy of current rotavirus vaccines in
India
There are no efficacy trials of the licensed
rotavirus vaccines available in India. The data from other developing
countries shows efficacy ranging from 17.6% (in Mali) to 61.2% (in South
Africa and Malawi) [5-9]. There is definite gradient in the efficacies
of these vaccines when different regions of the world are
compared-highest in US and Europe, moderate in Latin America, and low in
Africa and Asia (5-12). IAPCOI still believes that in developing
countries with high rotavirus disease incidence, even moderate to low
vaccine efficacy translates into significant numbers of severe rotavirus
gastroenteritis cases prevented and into significant public health
impact. More rotavirus deaths may be prevented in developing countries
despite lower vaccine efficacy than in countries with low rotavirus
disease burden and higher vaccine efficacy [13]. However, considering
that oral vaccines elicit diminished immune responses or have lower
efficacy in developing countries than in developed countries [14], and
since India is having history of poor performance of other oral
vaccines, notably OPV in recent past [15-17], it would not be prudent to
extrapolate data from other countries having comparable epidemiologic,
economic, and demographic indices.
3. Administration schedule of rotavirus
vaccines
In a recent community-based study from Vellore, it
was noted that rotavirus infection generally occurred early in life,
levels of re-infection were high and even three natural infections were
able to provide only 79% protection against moderate or severe disease,
with no evidence of homotypic protection as believed so far [18].
Therefore, there may be a need for modification of the rotavirus
vaccination strategy in India, by increasing the dose or increasing the
number of doses or delaying the doses or even considering neonatal
immunization. These considerations were further supported by the
immunogenicity study of another live attenuated human oral rotavirus
vaccine 116E in Indian infants, where administration of higher (1 × 10 4 ffu
Vs 1 × 105 ffu) and more
frequent (2 vs 3) doses resulted in more robust immune responses
[19]. Consequently, the ongoing phase III efficacy trial with this
strain is conducted with higher dose (105 ffu)
and a 3 dose schedule (6, 10 and 14 weeks) [19]. It can be argued that
one study in South Africa and Malawi with monovalent rotavirus vaccine
(RV1, marketed as Rotarix) did not detect significant differences in
vaccine immunogenicity or efficacy on pooled analysis between the cohort
receiving two vaccine doses and the cohort receiving three doses [7].
However, there was a slight but non-significant trend toward higher sero-conversion
rates and vaccine efficacy with the three-dose schedule, and these
differences were more marked in South Africa (81.5 [55.1–93.7] vs
72.2 [40.4–88.3]) than in Malawi (49.7 [11.3–72.2] vs 49.2
[11.1–71.7]) [7]. The two-dose schedule used in this trial was 10 and 14
weeks instead of 6 and 10 weeks [7].
Administering rotavirus vaccines at younger ages
could further lower the immunogenicity of the vaccines, because of the
potential for greater interference of maternal antibody and enhanced
replication of the oral poliovirus vaccine [7]. In the above African
study with RV-1, the researchers accepted that the study was not powered
to detect differences in dose schedule [7]. Furthermore, there have been
low seroconversion rates (58.3%; 95% CI: 48.7; 67.4) with two doses of
RV1 in comparison with three-dose schedule of RV5 (82.4% (CI; 75; 90%)
and 116E (89.7% (42.4; 80.6%) in immunogenicity studies in India
[19-21]. In the RV1 trial, the first dose was administered between 8-10
weeks (mean age-8.7 weeks) and the second dose between 12-16 weeks (mean
age-13.4 weeks) [20]. Hence, there is no immunogenicity data for 6 and
10 weeks administration or data on interference with simultaneous OPV
administration from India. It is important when examining immunogenicity
data to point out that although seroconversion is not a direct proxy for
efficacy, it does demonstrate that the virus is able to colonize the
infant gut and induce a robust immune response.
According to the WHO Ad-hoc Group of Experts on
rotavirus vaccines [22], most countries with high rotavirus disease
incidence or high under-5 mortality rates (where children would
particularly benefit from robust protection from rotavirus infection)
have 6, 10, 14 week EPI schedules. If rotavirus vaccines are to be
co-administered with OPV in a setting with an EPI vaccination schedule
beginning at 6 weeks of age, the second dose of RV1 may not be
sufficient to provide adequate immunity against severe rotavirus disease
[22]. A 2-dose schedule at 10 and 14 weeks is also assumed to be
programmatically problematic, since this would likely result in a
failure in administration of the full course of vaccines to children in
developing countries due to the restrictive upper age limit for
rotavirus vaccine administration, resulting from the approach of
attempting to avoid administration of rotavirus vaccines during the ages
when there is a heightened risk of intussusceptions [22]. After debating
intensely, the committee thinks that there is a need to seriously relook
at the proper administration schedule of rotavirus vaccines in India in
order to achieve higher yields in term of protective efficacy.
4. Homotypic vs. heterotypic protection
and potential impact of vaccination on Rotavirus strain diversity
Distribution of rotavirus genotypes exhibits
distinctive changes, both due to natural cyclical changes or due to
selective pressures imposed by vaccines. There is currently much
interest in elucidating the strain dynamics of rotavirus to determine
whether vaccination may lead to the replacement of vaccine-type strains.
According to a new modeling study, the predicted frequency of cycling
depends on the relative strength of homotypic vs. heterotypic
immunity. Vaccination that provides strong protection against G1 and
weaker protection against other strains will likely lead to an increase
in the relative prevalence of non-G1 strains, whereas a vaccine that
provides equally strong immunity against all strains may promote the
continued predominance of G1 [23]. Overall, however, disease incidence
is expected to be substantially reduced under both scenarios and remain
below pre-vaccination levels despite the possible emergence of new
strains. The committee concludes that better understanding of homotypic
vs. heterotypic immunity, both natural and vaccine-induced, will
be critical in deciding the inclusion of a particular rotavirus vaccine
in the national immunization program and predicting the impact of
vaccination. It also urges the need of effective strain monitoring
prospectively in different zones to determine changes in circulating
strains over a period of time.
5. Safety of rotavirus vaccines and
post-marketing surveillance data on acute intussusception in India
The committee reviewed the emerging data on
intussusception related to current rotavirus vaccines following
large-scale use of these vaccines in Mexico, Brazil, Australia and US
[24-27]. The post-marketing surveillance (PMS) data from India by the
manufacturers of two rotavirus vaccines licensed in India was also
reviewed.
Based on PMS data, the current rotavirus vaccines
have been associated with an increased risk of intussusceptions (about
1–2/100,000 infants vaccinated) for a short period after administration
of the first dose in some populations [24]. This risk is 5–10 times
lower than that observed with the previously licensed vaccine (1 case
per 10,000 doses). There are no published reports on incidence/rates of
acute intussusception following rotavirus vaccination in India. However,
the PMS data (unpublished) of Indian manufacturers revealed 13 cases of
acute intussusceptions associated (causality not yet proved) with
rotavirus vaccines administration since the launch of RV1 in India till
December 2011, and two cases following RV5 during a five-month
surveillance period (May-September 2011) in India.
There is limited information on the incidence of
intussusception and its risk factors in India. No large-scale trials of
rotavirus vaccines have been conducted in the country to assess whether
there is an increased risk of intussusception associated with the
vaccination. Data on background rates of intussusception in developing
countries are required to facilitate informed decision making about use
of new rotavirus vaccines. These background rates are also needed for
estimation of the sample size needed for studies to demonstrate safety
both before and after licensure of new rotavirus vaccines. Such
population-based data are not available in most developing countries,
including India. However, a recent study from Delhi found the incidence
of intussusception requiring hospitalization was 17.7 cases per 100,000
infant-years of follow-up (95% CI: 5.9-41.4 cases per 100,000
infant-years) [28]. The study also concluded that
natural rotavirus infection did not appear to be a major cause of
intussusception in Indian infants. This incidence appears to be lower
than that reported in other middle- and high-income countries. Another
retrospective study from a tertiary-care hospital from south India
identified 31 children with definite intussusception during the study
period of 1 January 2001-30 June 2004 [29].
After reviewing recent data, the committee concludes
that there is definite albeit a small risk of acute intussusceptions
following use of current generation of rotavirus vaccines. However, the
benefits of rotavirus vaccination against severe diarrhea and death from
rotavirus infection far exceed the miniscule risk of intussusceptions.
It urges the manufacturers to actively monitor the risk of
intussusceptions as the usage of these vaccines is bound to go up. This
will also require strengthening of AEFI surveillance in the country.
Information about the possible risk of intussusceptions associated with
rotavirus vaccination needs to be communicated clearly to the national
decision-makers, health-care providers, and parents. The committee also
stresses that while prescribing them in office practice; there is a need
to strictly adhere to the set upper age-limits, i.e. the first
dose of either RV1 or RV5 be administered between the ages of 6 weeks
and 14 weeks 6 days, and that the maximum age for administering the last
dose of either vaccine should be 32 weeks [30]. The committee has
recommended inclusion of the history of intussusception in the past as
an absolute contraindication for rotavirus vaccines (RV1 and RV5)
administration.
B. Hemophilus Influenzae Type B Vaccine
The committee discussed the recent reports on the
safety of Hib-containing pentavalent vaccines including a new PIL
against its introduction in two southern states [31, 32]. It also
reviewed the disease burden of Hib disease in India and PMS data on Hib
and Hib containing combination vaccines. The committee decided to
publish a detailed position paper on Hib-disease and Hib-vaccines.
According to PMS data of one Indian manufacturer, a total of 98 (46
serious and 49 non-serious) AEFI episodes have been reported for 53.51
million doses (overall frequency 1.83/million doses, and for serious
AEFI 0.85/million) from October 2004 through December 2011. The
committee expressed satisfaction on impressive performance of Hib and
Hib-containing vaccines as far as safety issues are concerned. The
committee concluded that there was no safety concerns of Hib vaccines as
reported frequently in lay media. It strongly supports the Government of
India’s efforts to introduce this vaccine in all the states of the
country.
C. Pneumococcal Conjugate Vaccines
1. Burden of pneumococcal diseases in India
The committee reviewed the available data on the
incidence of pneumococcal diseases (PD) in India and found that there
was no nationally representative study of pneumonia incidence from the
community. Most studies of severe pneumonia were hospital-based; hence,
may have missed cases. There were few older studies, based on parental
reporting of symptoms that again showed lower incidence. Most of the
available data on PD was from hospitals and on meningitis.
According to the WHO’s Child Health Epidemiology
Reference Group (CHERG) pneumonia working group, incidence of clinical
pneumonia among children <5 years in India for the year 2004, was
estimated to be 0.37 episodes per child year [33]. One Indian study
reported that the incidence of severe clinical pneumonia ranged from
0.03 to 0.08 per child-year at three study sites [34].
Another Indian study finds that Indian children <5 years of age suffer
~3 episodes of respiratory infection per year, with heavier burden on
younger children. Approximately, 1 in 5 episodes is a lower or severe
lower respiratory infection [35].
There is no systematic review or nation-wide study of
etiology of childhood pneumonia in India. The incidence of pneumonia
(ALRI) in India was found to be 290-536 and of severe pneumonia (severe
ALRI) was 27-96 per 1000 child-years India. Out of these cases, 18-59%
of all pneumonia (ALRI) and 53% of all severe pneumonia (severe ALRI)
were of bacterial origin [36]. Viruses mainly respiratory syncytial
virus (RSV), influenza A and B, para influenza 1, 2 and 3, and
adenovirus are responsible for 22.1% of under five year old children
patients with ARI, but only RSV and para-influenza 3 were seen to cause
severe ALRI disease [35]. Pneumococci accounted for 5-12% of all severe
pneumonia cases across studies; 12-30% of pneumonia cases with a
confirmed etiology [36]. A recent systematic review reported that about
12-35% of childhood pneumonias were caused by pneumococci and 10-15% by
H. influenzae and RSV each [37].
Another India-specific estimate for the year 2005
found 136,000 deaths (46,000-253,000) caused by pneumococcal diseases
comprising 10% of deaths in Indian children aged 1-59 months [38]. The
death rate for pneumococci was 106 per 100,000 (range 36-197), and more
than two-thirds of pneumococcal deaths were pneumonia-related. Central
and Eastern regions of the country had highest pneumococcal mortality
with more than half of all Indian deaths occurring in four states:
Bihar, Madhya Pradesh, Rajasthan, and Uttar Pradesh [38]. According to a
two year prospective study at three Bengaluru hospitals in south India,
incidence of invasive pneumococcal disease (IPD) in the first year of
study among less than 2-year old children was found to be 28.28 cases
per 100,000 population in which pneumonia contributed 15.91 and acute
bacterial meningitis (ABM) 6.82 cases [39].
There is also lack of community-based studies on
incidence of acute bacterial meningitis in India. There was only limited
data from prospective population-based incidence studies not only from
India but from entire Asia. A study from Vellore found an annual
incidence of ‘possible’, ‘probable’ and ‘proven’ ABM as 86, 37.4 and
15.9 per 100,000 children per year, respectively [40]. Assuming that the
probable and proven cases were truly ABM, the burden of disease was
53/100,000/year in under-five children [40]. According to the recent
review on epidemiology of pneumococcal infections in India, pneumococci
were responsible for 27-39% of all cases of ABM in children [36].
2. Distribution and prevalence of different pneumococcal
serotypes in India
The committee reiterated its stand on the
significance of knowing prevalence of distribution of different
pneumococcal serotypes in the community since each serotype had a
distinct ‘personality’ and represented a distinct disease.
The committee reviewed studies [41-49] on the
distribution and prevalence of different pneumococcal serotypes in the
country, including some recent studies done by vaccine manufacturers in
India like Pneumonet by M/s Pfizer [39] and Alliance for Surveillance of
Invasive Pneumococci (ASIP) by M/s GSK (unpublished). The committee
concluded that the data on prevalence of different pneumococcal
serotypes in the country was sparse and limited to few hospital based
studies. On the basis of available data, it is difficult to evaluate the
coverage of serotypes included in the existing Pneumococcal conjugate
vaccine (PCV) formulations. There were only handful of small
hospital-based studies mostly from south India [41, 43], and the only
comparatively large multi-centric study (Invasive Bacterial Infection
Surveillance (IBIS) multi-centric study from six centers across India in
1994-1997) was more than a decade old [42]; however, it is the one which
is most frequently cited. The large studies from Asian and other
neighboring countries like PneumoAdip [44], ANSORP [45, 46], SAPNA [47],
etc. did not have adequate representation of isolates from India.
Though a limited number of serotypes cause most
invasive pneumococcal disease (IPD) worldwide and the serotypes included
in existing PCV formulations responsible for 49%-88% of deaths in
developing countries of Africa and Asia where PD morbidity and mortality
are the highest [49], still there is a need of establishing a real-time
multi-site comprehensive pneumococcal disease surveillance including
both population and hospital-based surveillance arms. This ongoing
project should also include data on zonal distribution and prevalence of
different serotypes on annual basis. There is need to consolidate all
ongoing surveillance projects run by different vaccine manufacturers to
accord more credibility and avoid bias in the results. There is need to
incorporate more sophisticated diagnostic tests like
immune-chromatography (ICT), latex particle agglutination (LPA), and
real-time polymerase chain reaction (PCR) apart from cultures to
increase the yields. Since few serotypes are difficult to grow and under
diagnosed by culture (such as serotype 3), the PCR can be used to pick
serotypes from culture negative cases as done in few European countries
[50]. The surveillance should not be a one-time project but should be an
ongoing initiative to pick natural variations in the sero-epidemiology.
For example, in Bangladesh, there were differences in the serotypes
profile of hospital-based and population-based surveillance [51-53].
Further, the ongoing surveillance project picked a new serotype, type 2
as the predominant serotypes, not covered by the existing PCV
formulations [53]. Hence, surveillance should be prolonged enough to
pick the changing epidemiology over the years.
The surveillance project should have three important
objectives-to collect data on serotype distribution to guide appropriate
pneumococcal conjugate vaccine formulations, to identify trend of
antimicrobial resistance amongst different serotypes, and lastly,
to assess the impact of vaccine introduction (in national immunization
program [NIP] on the serotype distribution and replacement, if any. The
committee urges the Government of India (GoI) to take the initiative and
launch this project all over the country.
3. Suitability of PCV13 vs PCV10 for
Indian children
The committee studied the recent data on PCV13 and
PCV10. The committee also reviewed the reports of PCV13 studies done
worldwide on immune responses (IgG - GMC, OPA – GMT) and boostability
for the serotype 3 capsular antigen [54], and the immune responses
following post-primary and post-booster series against serotype 19A
infections, with PCV10 and PCV13 [55, 56]. It has reviewed the interim
data of COMPAS trial done in three Latin American countries with PCV10
[57] and effectiveness of PCV 10 in Brazil [58].
The committee also reviewed available data on the
efficacy of the new serotypes in the PCV13. In England and Wales [59],
vaccine effectiveness (VE) for the new serotypes for 2 doses under a
year was 78% (95% CI -18-96%) and 77% (CI: 38-91%) for one dose over a
year. VE for 7F and 19A was 76% (CI: 21-93%) and 70% (CI: 10-90%),
respectively for one or more than one dose, for serotypes 1 and 3 was
62% and 66%, respectively although confidence intervals spanned zero.
IPD due to PCV13-only serotypes halved in children under 2 years in the
study period [59].
The committee believes that the direct protection
rendered by the serotype included in a vaccine formulation is definitely
superior to any cross protection offered by the unrelated serotypes even
of the same group in a PCV formulation. However, the committee is not
convinced about the clinical efficacy of serotype 3 contained in PCV13
despite multiple studies showing good functional immune responses after
the infant series and reasonably good effectiveness. There has been no
consistent PCV13 impact on serotype 3 IPD or carriage reported so far.
Similarly, the committee thinks that despite using a
different conjugation method (cyanylation versus reductive
amination) [60], PCV10 is yet to demonstrate a better clinical efficacy
(cross protection) against serotype 19A than shown by PCV7. Though
current seroprevalence of type 19A in India is not known, but its
presence is confirmed by almost all the recent studies [39, 45, 46].
Since this serotype is increasing in many other Asian countries and has
got higher antimicrobial resistance characteristics than other serotypes
[45, 46], the committee believes that protection against 19A will be
critical to determine which vaccine is appropriate to use in the
country. Recent data has now shown that PCV13 provides protection
against 19A [59], while it is unknown if the presence of ‘novel’ 19F in
PCV10 will provide cross protection against 19A [61]. On the other hand,
the committee is convinced about the adequate cross-protection rendered
by serotype 6B to 6A based on performance of PCV7 in many European
countries and US in decreasing IPDs caused by 6A. However, the exact
role and significance of 6C which is clearly emerging as replacement
serotype is yet to be determined.
The committee thinks that though non-typeable
Haemophilus influenzae (NTHi), a co-pathogen plays some role in the
pathogenesis of mucosal disease with Streptococcal pneumoniae,
its role in childhood pneumonia is still not proven.
After appraising in detail all the available relevant
data, the committee concludes that since there is scarcity of data on
the prevalence of pneumococcal serotypes including serotypes 3, 6A and
19A, and NTHi in India, it is almost impossible to comment on the exact
superiority of one product over other. Further, in the absence of head
to head trials, it is difficult to determine if either vaccine has a
clear advantage over other. Although recent publications [49] state that
the same few serotypes are responsible for a large proportion of PD in
all geographic regions and new PCVs cover almost 70% of serotypes
prevailing in India, the committee believes that it is critical to know
what percentage of pneumonia, meningitis and other IPDs are caused by
the pneumococcal serotypes not included in existing formulations.
4. Recommendations for premature and low birth weight infants
The committee has now stressed the need of treating
prematurity and very-low birth weight (VLBW) infants as another high
risk category for pneumococcal vaccination. VLBW infants have up to
9-fold higher incidence of invasive pneumococcal diseases (IPD) as
compared to full size babies [62]. The risk ratio for LBW infants
compared with normal birth weight infants was 2.6, and for premature
infants compared with full-term infants was 1.6 [62]. PCV must be
offered to these babies on priority basis. PCV was as immunogenic in low
birth weight and preterm infants as in normal birthweight and fullterm
infants; the vaccine efficacy for both groups was found 100% [62].
Recommendations for IAP Immunization Timetable, 2012
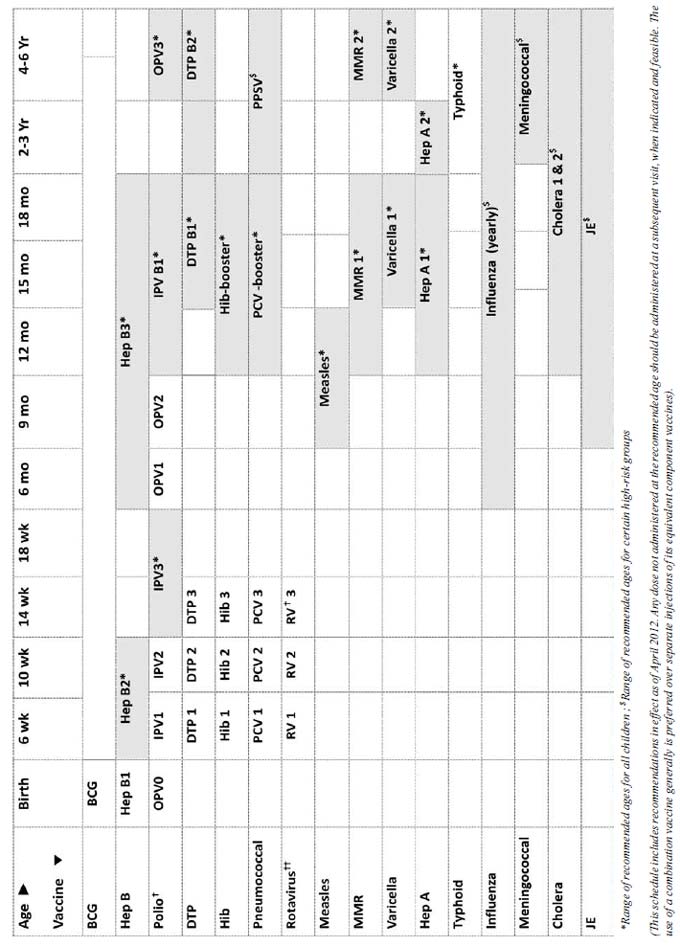
The IAPCOI has issued recommendations for the IAP
Immunization Timetable (Table I, Fig. 1,2) for the
year 2012 that includes the following major changes from last year:
TABLE I IAP Immunization Timetable 2012 (IAP recommended vaccines for routine use)
|
Age |
Vaccines |
Comments |
|
Birth |
BCG, OPV 0, Hep-B 1 |
Hepatitis-B: Administer Hep-B vaccine to all
newborns before hospital discharge. |
|
6 weeks |
DTwP 1/DTaP 1, IPV 1, Hep-B 2, Hib 1, Rotavirus 1, PCV 1 |
Polio: All doses of IPV may be replaced with
OPV if former is unaffordable/ unavailable; Additional doses of
OPV on all "Supplementary immunization activities" (SIAs);
Two doses IPV instead of 3 for primary series if started at 8
weeks, and 8 weeks interval between the doses.
Rotavirus: 2 doses of RV-1 (monovalent)
and 3 doses of RV-5 (pentavalent).
|
|
10 weeks |
DTwP 2/DTaP 2,
IPV 2, Hib 2, Rotavirus 2, PCV 2 |
|
|
14 weeks |
DTwP 3/DTaP 3,
IPV 3, Hib 3, Rotavirus 3, PCV 3 |
Rotavirus: Only 2 doses of RV1 are
recommended at present.
|
|
6 months |
OPV 1, Hep-B 3 |
Hepatitis-B: The final (third or fourth) dose
in the HepB vaccine series should be administered no earlier
than age 24 weeks and at least 16 weeks after the first dose. |
|
9 months |
OPV 2, Measles |
|
|
12 months |
Hep-A 1 |
Hepatitis A: For both killed and live
hepatitis-A vaccines 2 doses are recommended. |
|
15 months |
MMR 1,Varicella 1, PCV booster |
Varicella: The risk of breakthrough varicella
is lower if given 15 months onwards. |
|
16 to 18 months |
DTwP B1/DTaP B1,
IPV B1, Hib B1 |
The first booster (4th dose) may be administered as early as age
12 months, provided at least 6 months have elapsed since the
third dose. |
|
18 months |
Hep-A 2 |
Hepatitis A: For both killed and live
hepatitis-A vaccines, 2 doses are recommended.
|
|
2 years |
Typhoid 1 |
Typhoid: Typhoid revaccination every 3
years, if Vi-polysaccharide vaccine is used. |
|
4 ½ to 5 years |
DTwP B2/DTaP B2, OPV 3, MMR 2, Varicella 2, Typhoid 2 |
MMR: The 2nd dose can be given at anytime 4-8
weeks after the 1st dose. Varicella: The 2nd dose can be given
at anytime 3 months after the 1st dose. |
|
10 to 12 years |
Tdap/Td |
Tdap: Preferred to Td followed by Td every 10
years. |
|
HPV
|
HPV: Only for females, 3 doses at 0, 1-2
(depending on brands) and 6 months. |
IAP recommended vaccines for High-risk* children
(Vaccines under special circumstances): 1. Influenza
Vaccine, 2. Meningococcal Vaccine, 3. Japanese Encephalitis
Vaccine, 4. Cholera Vaccine, 5. Rabies Vaccine, 6. Yellow Fever
Vaccine, 7. Pneumococcal Polysaccharide vaccine (PPSV 23).
*High-risk category of children:
•Congenital or acquired immunodeficiency (including HIV
infection)
•Chronic cardiac, pulmonary (including asthma if treated with
prolonged high-dose oral corticosteroids), hematologic, renal
(including nephrotic syndrome), liver disease and diabetes
mellitus
•Children on long term steroids, salicylates, immunosuppressive
or radiation therapy
•Diabetes mellitus, Cerebrospinal fluid leak, Cochlear
implant, Malignancies
•Children with functional/anatomic asplenia/hyposplenia
•During disease outbreaks
•Laboratory personnel and healthcare workers
•Travelers |
 |
| |
|
Fig.1 IAP Recommended immunization
schedule for children aged 0-6 years (with range), 2012.
|
|
1. BCG Vaccine
• Should be given at birth or at first
contact • Catch up may be given up to 5 years
2. Hepatitis B (HepB) vaccine
• Minimum age: birth • Administer monovalent
HepB vaccine to all newborns before hospital discharge • Mono-
valent HepB vaccine should be used for doses administered before
age 6 weeks • Administration of a total of 4 doses of HepB
vaccine is permissible when a combination vaccine containing
HepB is administered after the birth dose • Infants who did
not receive a birth dose should receive 3 doses of a HepB
containing vaccine starting as soon as feasible • The ideal
minimum interval between dose 1 and dose 2 is 4 weeks, and
between dose 2 and 3 is 8 weeks • Ideally, the final (third or
fourth) dose in the HepB vaccine series should be administered
no earlier than age 24 weeks and at least 16 weeks after the
first dose • Hep B vaccine may also be given in any of the
following schedules: Birth, 1, & 6 mo, Birth, 6 and 14 weeks; 6,
10 and 14 weeks; Birth, 6 weeks,10 weeks, 14 weeks, etc.
3. Poliovirus vaccines †
• OPV in place of IPV If IPV is
unaffordable/unavailable, minimum 3 doses • Additional doses of
OPV on all SIAs • IPV: Minimum age: 6 weeks • IPV: 2 instead of
3 doses can be also used if primary series started at 8 weeks
and the interval between the doses is kept 8 weeks • IPV
catch-up schedule: 2 doses at 2 months apart followed by a
booster after 6 months
4. Diphtheria and tetanus toxoids and
pertussis (DTP) vaccine
• Minimum age: 6 weeks • The first booster
(4thth dose) may be administered as early as age 12 months,
provided at least 6 months have elapsed since the third dose • DTwP/DTaP/Tdap/Td:
Catch up below 7 years: DTwP/DTaP at 0, 1 and 6 months; • Catch
up above 7 years: Tdap, Td, Td at 0, 1 and 6 months.
5. Haemophilus influenzae type b (Hib)
conjugate vaccine
• Minimum age: 6 weeks • Catch up in 6-12
months; 2 doses 1 month apart and 1 booster; 12-15 months: 1
primary and 1 booster; above 15 months single dose.
6. Pneumococcal vaccines
• Minimum age: 6 weeks for pneumococcal
conjugate vaccine [PCV]; 2 years for pneumococcal polysaccharide
vaccine [PPSV] • Administer 1 dose of PCV to all healthy
children aged 24 through 59 months who are not completely
vaccinated for their age • For children who have received an
age-appropriate series of 7-valent PCV (PCV7), a single
supplemental dose of 13-valent PCV (PCV13) is recommended for:
• All children aged 14 through 59
months • Children aged 60 through 71 months with underlying
medical conditions • Administer PPSV at least 8 weeks after last
dose of PCV to children aged 2 years or older with certain
underlying medical conditions (certain high-risk • PCV: Catch up
in 6-12 months: 2 doses 1 month apart and 1 booster; 12-23
months: 2 doses 2 months apart; 24 mo & above: single
dose • PPSV: Revaccination only once after 3-5 years only in
certain high risk patients.
7. Rotavirus (RV) vaccines ††
• Minimum age: 6 weeks for both RV-1 [Rotarix]
and RV-5 [Rota Teq]) • Only two doses of RV-1 are recommended at
present • The maximum age for the first dose in the series is 14
weeks, 6 days; and 8 months, 0 days for the final dose in the
series • Vaccination should not be initiated for infants aged 15
weeks, 0 days or older.
8. Measles
• Minimum age: At completed months/270
completed days • Catch up vaccination beyond 12 months should be
MMR • Measles vaccine can be administered to infants aged 6
through 11 months during outbreaks. These children should be
revaccinated with 2 doses of measles containing vaccines, the
first at ages 12 through 15 months and at least 4 weeks after
the previous dose, and the second at ages 4 through 6 years.
9. Measles, mumps, and rubella (MMR)
vaccine
• Minimum age: 12 months • The second dose
may be administered before age 4 years, provided at least 4
weeks have elapsed since the first dose.
10. Varicella vaccine
• Minimum age: 12 months • The risk of
breakthrough varicella is lower if given 15 months onwards • The
second dose may be administered before age 4 years, provided at
least 3 months have elapsed since the first dose • For children
aged 12 months through 12 years, the recommended minimum
interval between doses is 3 months. However, if the second dose
was administered at least 4 weeks after the first dose, it can
be accepted as valid.
11. Hepatitis A (HepA) vaccine
• Minimum age: 12 months • Two doses of both
killed and live HepA vaccines • Administer the second (final)
dose 6 to18 months after the first.
12. Typhoid vaccine
• Only Vi-PS (polysaccharide) vaccine is
recommended • Minimum age: 2 years; Revaccination every 3
years • Vi-PS conjugate vaccine: data not sufficient to
recommend for routine use of currently available vaccine
13. Influenza vaccine
• Minimum age: 6 months for trivalent
inactivated influenza vaccine • First time vaccination: 6 months
to below 9 years: two doses 1 month apart; 9 years and above
single dose; Annual revaccination with single dose • For
children aged 6 months to below 9 years: For the 2012 season,
administer 2 doses (separated by at least 4 weeks) to those who
did not receive at least 1 dose of the 2010–11 vaccine. Those
who received at least 1 dose of the 2010–11 vaccine require 1
dose for the 2011–12 season • Best time to vaccinate: as soon as
the new vaccine is released and available in the market & just
before the onset of rainy season;
14. Meningococcal vaccine
• Only meningococcal polysaccharide vaccine
(MPSV) is available • Minimum age: 2 years • Revaccination only
once after 3 years in those at continued high risk
15. Cholera Vaccine
• Minimum age: one year (killed whole cell
vibrio cholera (Shanchol) • Two doses 2 weeks apart for >1 year
old
16. Japanese encephalitis (JE) vaccine
• Recommended in endemic areas only • Live
attenuated, cell culture derived SA-14-14-2 vaccine is
preferred • Minimum age: 8 months; can be co-administered with
measles vaccine at 9 months; single dose • Catch up vaccination:
all susceptible children up to 15 yrs should be administered
during disease outbreak/ahead of anticipated outbreak in
campaigns.
|
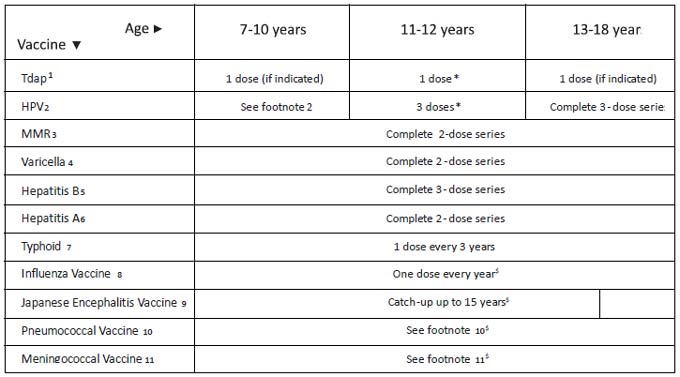 |
|
Range of recommended ages for all children;
*Range of recommended ages for catch-up immunization; $Range of
recommended ages for certain high-risk groups.
Fig. 2 IAPCOI recommended immunization
schedule for persons aged 7 through 18 years, 2012 (with range).
1. Tetanus and diphtheria toxoids and
acellular pertussis (Tdap) vaccine: • Minimum age: 10 years for
Boostrix and 11 years for Adacel • Persons aged 11 through 18
years who have not received Tdap vaccine should receive a dose
followed by tetanus and diphtheria toxoids (Td) booster doses
every 10 years thereafter • Tdap vaccine should be substituted
for a single dose of Td in the catch-up series for children aged
7 through 10 years • Tdap vaccine can be administered regardless
of the interval since the last tetanus and diphtheria toxoid–containing
vaccine • Catch up above 7 years: Tdap, Td, Td at 0, 1 and 6
months • Tdap can also be administered safely to pregnant women.
2. Human papillomavirus (HPV)
vaccines: • HPV4 [Gardasil] and HPV2 [Cervarix] • Minimum age: 9
years • Either HPV4 (0, 2, 6 months) or HPV2 (0, 1, 6 months) is
recommended in a 3-dose series for females aged 11 or 12
years • HPV4 can also be given in a 3-dose series for males aged
11 or 12 years • The vaccine series can be started beginning at
age 9 years • Administer the second dose 1 to 2 months after the
first dose and the third dose 6 months after the first dose (at
least 24 weeks after the first dose).
3. Measles, mumps, and rubella (MMR)
vaccine: • The minimum interval between the 2 doses of MMR
vaccine is 4 weeks • One dose if previously vaccinated with one
dose.
4. Varicella (VAR) vaccine: • For persons
without evidence of immunity, administer 2 doses if not
previously vaccinated or the second dose if only 1 dose has been
administered • For persons aged 7 through 12 years, the
recommended minimum interval between doses is 3 months. However,
if the second dose was administered at least 4 weeks after the
first dose, it can be accepted as valid • For persons aged 13
years and older, the minimum interval between doses is 4 weeks.
5. Hepatitis B (HepB) vaccine: • Administer
the 3-dose series to those not previously vaccinated • For those
with incomplete vaccination, the recommended minimum interval
between dose 1 and dose 2 is 4 weeks, and between dose 2 and 3
is 8 weeks. The final (third or fourth) dose in the HepB vaccine
series should be administered at least 16 weeks after the first
dose.
6. Hepatitis A (Hep A) vaccine: • Administer
2 doses at least 6 months apart to unvaccinated persons • For
catch up vaccination, pre vaccination screening for Hepatitis A
antibody is recommended in children older than 10 years as at
this age the estimated sero-positive rates exceed
50% • Combination of Hep B and Hep A may be used in 0, 1, 6
schedule.
7. Typhoid vaccine : • Only Vi-PS
(polysaccharide) vaccine is recommended • Vi-PS conjugate
vaccine: data not sufficient to recommend for routine use of
currently available vaccine • A minimum interval of 3 years
should be observed between 2 doses of typhoid vaccine.
8. Influenza Vaccine : • Administer 1 dose to
persons aged 9 years and older • For children aged 6 months
through 8 years • For the 2012 season, administer 2 doses
(separated by at least 4 weeks) to those who did not receive at
least 1 dose of the 2010-11 vaccine. Those who received at least
1 dose of the 2010-11 vaccine require 1 dose for the 2011–12
season • Annual revaccination with single dose • Best time to
vaccinate: as soon as the new vaccine is released and available
in the market & just before the onset of rainy season;
9. Japanese Encephalitis (JE) Vaccine :
• Only in endemic area as catch up • Currently no type of JE
vaccine available in private Indian market • Live attenuated,
cell culture derived SA-14-14-2 JE vaccine should be
preferred • Dose: 0.5 ml, SC, single dose up to 15 yrs.
10. Pneumococcal Vaccines : • Pneumococcal
conjugate vaccine [PCV] and pneumococcal polysaccharide vaccine
[PPSV] both are used in certain high risk group of children • A
single dose of PCV may be administered to children aged 6
through 18 years who have anatomic/functional asplenia, HIV
infection or other immunocompromising condition, cochlear
implant, or cerebral spinal fluid leak • Administer PPSV at
least 8 weeks after the last dose of PCV to children aged 2
years or older with certain underlying medical conditions,
including a cochlear implant • A single re-vaccination (with
PPSV) should be administered after 5 years to children with
anatomic/functional asplenia or an immunocompromising condition.
11. Meningococcal Vaccine: • Recommended
only for certain high risk group of children, during outbreaks,
travelers to endemic areas, and students going for study
abroad; • Only meningococcal
polysaccharide vaccine (MPSV) is available; • Minimum
age: 2 years; • Dose
schedule: a single dose 0.5 ml SC/ IM is recommended; • Revaccination
only once after 3 yrs in those at continued high risk.
|
A. Poliovirus immunization
In the light of remarkable achievement in the field
of polio eradication in India over the last one year [63], the committee
has now decided to adopt a sequential IPV-OPV schedule. This will pave
the way to ultimate adoption of all-IPV schedule in future considering
the inevitable cessation of OPV from immunization schedules owing to its
safety issues (VAPP and cVDPVs). This policy is in accordance with the
recent decision taken by GPEI where phased removal of Sabin viruses,
beginning with highest risk (type 2) would be undertaken [64]. This will
result in elimination of VDPV type 2 in ‘parallel’ with eradication of
last wild polioviruses by switching from tOPV to bOPV for routine EPI
and campaigns. This switch will result in much early introduction of IPV
than anticipated, at least in high risk areas for VDPVs, to provide type
2 protection [64].
There is considerable evidence to show that
sequential schedules that provide IPV first, followed by OPV, can
prevent VAPP while maintaining the critical benefits conferred by OPV (i.e.,
high levels of gut immunity). Data from several studies show that
sequential schedules considerably decrease the risk of VAPP [65-68].
There is moderate level of scientific evidence that sequential
immunization schedules starting with two or more doses of IPV and
followed by two or more doses of OPV(at an interval of 4-8 weeks) induce
protective immunological responses to all three poliovirus serotypes in
more than 90% of vaccines [69]. However, the committee has retained the
birth dose of OPV as recommended earlier. Providing the first OPV dose
at a time when the infant is still protected by maternally-derived
antibodies may, at least theoretically, also prevent VAPP. A birth dose
of OPV is considered necessary in countries where the risk of poliovirus
transmission is high [70].
The primary schedule
The committee recommends birth dose of OPV, three
primary doses of IPV at 6, 10 and 14 weeks, followed by two doses of OPV
at 6 and 9 months, another dose (booster) of IPV at 15-18 months and OPV
at 5 yrs. Alternatively, two doses of IPV can be used for primary series
at 8 and 16 weeks, though this schedule is immunologically superior to
EPI schedule and the number of IPV doses is reduced, but will be more
cumbersome due to extra visits and incompatibility with combination
formulations. Further, the child would be susceptible to WPV infection
for the first two months of life considering the epidemiology of WPV in
India till quite recently.
Since IPV administered to infants in EPI schedule
(i.e. 6 weeks, 10 weeks and 14 weeks) results in suboptimal
seroconversion [70], hence, a supplementary dose of IPV is recommended
at 15-18 months. IPV should be given intramuscularly (preferably) or
subcutaneously and may be offered as a component of fixed combinations
of vaccines. However, the committee recommends that if IPV is
unaffordable or unavailable, the primary series must be completed with
three doses of OPV given at 6, 10, and 14 weeks. No child should be left
without adequate protection against wild polio virus (i.e. three
doses of either vaccine). All OPV doses (mono-, bi- or trivalent)
offered through supplemental immunization activities (SIAs), should also
be provided.
Catch-up schedule
IPV may be offered as ‘catch up vaccination’ for
children less than 5 years of age who have completed primary
immunization with OPV. IPV can be given as three doses; two doses at two
months interval followed by a third dose after 6 months. This schedule
will ensure a long lasting protection against poliovirus disease.
Recommendations for travelers
The committee has now issued the following
recommendations for travelers to polio-endemic countries or areas:
• For those who have previously received at least
3 doses of OPV or IPV should be offered another dose of polio
vaccine as a once-only dose before departure.
• Non-immunized individuals should complete a
primary schedule of polio vaccine, using either IPV or OPV. Primary
series includes at least three doses of either vaccine.
• For people who travel frequently to
polio-endemic areas but who stay only for brief periods, a one-time
only additional dose of a polio vaccine after the primary series
should be sufficient to prevent disease [70].
B. Hepatitis B immunization
The committee has now recommended the following
schedule for routine Hepatitis-B vaccination in office practice for
children: the first dose of a three-dose schedule should be administered
at birth, second dose at 6 weeks, and third dose at 6 months (i.e.
0–6 week–6 month). This schedule is not only more closer to
immunologically ideal and most widely used 0-1-6 months schedule, but
also confirms to latest ACIP recommendations wherein the final (third or
fourth) dose in the Hepatitis-B vaccine series should be administered no
earlier than age 24 weeks and at least 16 weeks after the first dose
[71]. It will replace the existing schedule of 0–6 week–14 week.
However, the Hepatitis-B vaccine may be given through other schedules,
considering the programmatic implications and logistic issues. The
committee stresses the significance and need of birth dose.
C. Influenza vaccination
The committee reviewed the WHO recommendations
regarding composition of flu vaccines for the southern and northern
hemisphere for use in the 2012-2013 influenza seasons [72-73]. For the
northern hemisphere, it will contain the following strains: an
A/California/7/2009 (H1N1) pdm09-like virus; an A/Victoria/361/2011
(H3N2)-like virus; and a B/Wisconsin/1/2010-like virus [72]. The last
two strains will be different from the last year’s vaccine for the
region; however, there will be no change in the composition of influenza
vaccines for the southern hemisphere for 2012 [73]. Last year, the
strains were similar for both the hemispheres. This will have impact on
the types of vaccines to be used in coming season.
As far as the influenza virus circulation in India is
concerned, the data since 2004 suggests a clear peaking of circulation
during the rainy season across the country- ‘June to August’ in north
(Delhi), west (Pune) and east (Kolkata), and ‘October to December’ in
south (Chennai) [74]. This data is also consistent with the WHO
circulation patterns for 2010 and 2011 for India which also shows a
clear peak coinciding with the rainy season across the country. These
data illustrate the difficulty in having effective uniform vaccination
timing for a vast country like India and have implications when
formulating vaccination policies. The evidence of antigenic drifts of
circulating influenza viruses in India, together with the temporal peaks
in seasonality of influenza in different parts of the country;
illustrate the need for a staggered approach in vaccination timing.
Hence, the best time for offering vaccine for individuals residing in
southern states would be just before the onset of rainy season, i.e.
before October while for rest of the country, it should be before June.
Though, the committee acknowledges that this issue is still contentious
and unresolved.
This is to be noted that WHO convenes two meetings to
provide recommendations for the usage of influenza vaccine in February
and September each year. The vaccine for the February recommendations
(Northern hemisphere) and September recommendations (Southern
hemisphere) becomes available after 6 months of each recommendation.
With the above background the vaccine that shall be available in
March-April 2012 (Southern hemisphere) this year is based on the
recommendation made in September 2011 which took into account the data
from the past year i.e. August 2010 to Sept 2011 (thus covering India’s
rainy season peak last year from June to August 2011). Whereas the
vaccine that shall be available in August 2012 (Northern hemisphere,
with the 2 new strains) shall be based on the recommendation made in
February 2012 which took into account the data from the past year i.e.
March 2011 to Feb 2012 which means that by the time it is available in
August 2012, the most of the country barring southern states may have
already passed the peak influenza activity.
In addition to this, WHO classifies India under the
‘South Asia’ transmission zone of Influenza circulation. This along with
summary review of the 2011 southern hemisphere winter influenza season
[73] strongly points India’s alignment with the availability of Southern
hemisphere vaccine (March-April) to ensure we have the latest available
strains for early vaccination to prevent the peak of circulation of
Influenza in the rainy season across the country.
D. Updated and consolidated footnotes of all IAPCOI
recommended vaccines
The committee has decided to update and consolidate
all the footnotes of IAP recommended vaccines. The readers can access
them at the committee’s official website at www.iapcoi.com
Funding: None; Competing interest:
None stated.
Writing committee: Committee on
Immunization, Indian Academy of Pediatrics 2011-13.
Annexure 1
Participants
IAPCOI members: TU Sukumaran; Rohit Agrawal; Vipin M
Vashishtha; A Parthasarathy; Nitin Shah; Raju Shah; Naveen Thacker;
Panna Choudhury; Suhas Prabhu; SG Kasi; S Sanjay; AJ Chitkara; Monjori
Mitra; Vijay Yewale and Pravin Mehta (Rapporteur).
Following were the special invitees who attended the
meeting during their respective sessions only:
Ashish Bavdekar, Pune; Krishna Ella (Bharat Biotech);
Sai D Prasad (Bharat Biotech); Shailesh Mehta (GSK vaccines); Swashraya
Shah (MSD); Sudhanshu Pandey (MSD); Rohit Arora (Sanofi Pasteur); Shafi
Kolhapure (Chiron Panacea); Gautam Rambhad (Wyeth).
|
Major Changes in Recommendations for IAP Immunization Timetable,
2012
• Polio: Sequential IPV-OPV schedule
is recommended for primary polio immunization in place of
combined OPV+IPV schedule.
• Hepatitis-B: ‘Birth-6 weeks-6
months’ is recommended as most preferred schedule instead of
earlier ‘0- 6 weeks-14 weeks’ schedule.
• History of intussusception in the past is
added as an absolute contraindication for rotavirus vaccine
administration.
• Prematurity and very-low birth weight are
added as another high risk category for pneumococcal
vaccination.
• Guidelines are provided for influenza vaccination.
|
| |
References
1. Indian Academy of Pediatrics Committee on
Immunization (IAPCOI). Consensus recommendations on immunization, 2008.
Indian Pediatr. 2008;45:635-48.
2. Tate JE, Burton AH, Boschi-Pinto C, Steele AD,
Duque J, Parashar UD. The WHO-coordinated Global Rotavirus Surveillance
Network. 2008 estimate of worldwide rotavirus-associated mortality in
children younger than 5 years before the introduction of universal
rotavirus vaccination programmes: a systematic review and meta-analysis.
Lancet Infect Dis. 2012;12:136-41.
3. Ramani S, Kang G. Burden of disease & molecular
epidemiology of group A rotavirus infections in India. Indian J Med Res.
2007;125:619-32.
4. Kang G, Arora R, Chitambar SD, Deshpande J, Gupte
MD, Kulkarni M, et al. Multicenter, hospital-based surveillance
of rotavirus disease and strains among indian children aged <5 years. J
Infect Dis. 2009;200:S147-53.
5. Phua KB, Lim FS, Lau YL, Nelson EA, Huang LM, Quak
SH, et al. Safety and efficacy of human rotavirus vaccine during
the first 2 years of life in Asian infants: randomised, double-blind,
controlled study. Vaccine. 2009;27: 5936-41.
6. Armah GE, Sow SO, Breiman RF, Dallas MJ, Tapia MD,
Feikin DR, et al. Efficacy of pentavalent rotavirus vaccine
against severe rotavirus gastroenteritis in infants in developing
countries in sub-Saharan Africa: a randomised, double-blind,
placebo-controlled trial. Lancet. 2010;376:606-14.
7. Madhi SA, Cunliffe NA, Steele D, Witte D, Kirsten
M, Louw C, et al. Effect of human rotavirus vaccine on severe
diarrhea in African infants. N Engl J Med. 2010;362:289-98.
8. Zaman K, Dang DA, Victor JC, Shin S, Yunus M,
Dallas MJ, et al. Efficacy of pentavalent rotavirus vaccine
against severe rotavirus gastroenteritis in infants in developing
countries in Asia: a randomised, double-blind, placebo-controlled trial.
Lancet. 2010;376:615-23.
9. Rotavirus vaccines: an update. Weekly Epidemiol
Rec. 2009;84:533-40.
10. Vesikari T, Matson DO, Dennehy P, Van Damme P,
Santosham M, Rodriguez Z, et al. Safety and efficacy of a
pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J
Med. 2006;354:23-33.
11. Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR,
Abate H, Breuer T, Clemens SC, et al. Safety and efficacy of an
attenuated vaccine against severe rotavirus gastroenteritis. N Engl J
Med. 2006;354:11-22.
12. Vesikari T, Karvonen A, Prymula R, Schuster V,
Tejedor JC, Cohen R, et al. Efficacy of human rotavirus vaccine
against rotavirus gastroenteritis during the first 2 years of life in
European infants: randomised, double-blind controlled study. Lancet.
2007;370:1757-63.
13. Rotavirus Vaccines. In: Yewale V,
Choudhury P, Thacker N, Editors. IAP Guide Book on Immunization. 6th
Edition. Mumbai: 2011. p. 116-23.
14. Levine MM. Immunogenicity and efficacy of oral
vaccines in developing countries: lessons from a live cholera vaccine.
BMC Biol. 2010;8:129.
15. Grassly NC, Fraser C, Wenger J, Deshpande JM,
Sutter RW, Heymann DL, Aylward RB. New strategies for the elimination of
polio from India. Science. 2006;314:1150-3.
16. John TJ. Antibody response of infants in tropics
to five doses of oral polio vaccine. Br Med J. 1976;1:812.
17. Paul Y. Oral polio vaccines and their role in
polio eradication in India. Expert Rev Vaccines. 2009;8:35-41.
18. Gladstone BP, Ramani S, Mukhopadhya I, Muliyil J,
Sarkar R, Rehman AM, et al. Protective effect of natural
rotavirus infection in an Indian birth cohort. N Engl J Med.
2011;365:337-46.
19. Bhandari N, Sharma P, Taneja S, Kumar T,
Rongsen-Chandola T, Appaiahgari MB, et al. A dose-escalation
safety and immunogenicity study of live attenuated oral rotavirus
vaccine 116E in infants: a randomized, double-blind, placebo-controlled
trial. J Infect Dis. 2009;200: 421-9.
20. Narang A, Bose A, Pandit AN, Dutta P, Kang G,
Bhattacharya SK, et al. Immunogenicity, reactogenicity and safety
of human rotavirus vaccine (RIX4414) in Indian infants. Hum Vaccin.
2009;5:414-9.
21. Safety, tolerability and immunogenicity of
vaccination with Rotateq in healthy infants in India. Study NCT00496054.
Available from:
http://clinicaltrials.gov/ct2/show/results/NCT00496054?sect=X0125#all
Accessed on March 9, 2012.
22. Detailed Review Paper on Rotavirus Vaccines by
Ad-hoc group of experts on rotavirus vaccines. Available from:
http://www.who.int/immunization/sage/3_Detailed_Review_Paper_on_Rota_Vaccines_17_3_2009.pdf
Accessed on February 8, 2012.
23. Pitzer VE, Patel MM, Lopman BA, Viboud C,
Parashar UD, Grenfell BT. Modeling rotavirus strain dynamics in
developed countries to understand the potential impact of vaccination on
genotype distributions. Proc Natl Acad Sci USA. 2011;108:19353-8.
24. World Health Organization. Rotavirus vaccine and
intussusception: report from an expert consultation. Weekly Epidemiol
Rec. 2011; 86: 317–324.
25. Patel MM, López-Collada VR, Bulhões MM, De
Oliveira LH, Bautista Márquez A, Flannery B, et al.
Intussusception risk and health benefits of rotavirus vaccination in
Mexico and Brazil. N Engl J Med. 2011;364:2283-92.
26. Buttery JP, Danchin MH, Lee KJ, Carlin JB,
McIntyre PB, Elliott EJ, Booy R, et al. Intussusception following
rotavirus vaccine administration: postmarketing surveillance in the
National Immunization Program in Australia. Vaccine. 2011;29:3061-6.
27. Baggs J, Haber P. Continued surveillance for
intussusception (IS) following RotaTeq in VAERS and VSD. Atlanta, GA,
Centers for Disease Control and Prevention , 2010
(http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-oct10/12-4-rota-VAERSRotaTeq.pdf,
accessed July 2011).
28. Bahl R, Saxena M, Bhandari N, Taneja S, Mathur M,
Parashar UD, et al. Population-based incidence of intussusception
and a case-control study to examine the association of intussusception
with natural rotavirus infection among Indian children. J Infect Dis.
2009;200:S277-81.
29. Bhowmick K, Kang G, Bose A, Chacko J, Boudville
I, Datta SK, et al. Retrospective surveillance for
intussusception in children aged less than five years in a South Indian
tertiary-care hospital. J Health Popul Nutr. 2009;27:660-5.
30. Cortese MM, Parashar UD. Centers for Disease
Control and Prevention (CDC). Prevention of rotavirus gastroenteritis
among infants and children: recommendations of the Advisory Committee on
Immunization Practices (ACIP). MMWR Recomm Rep. 2009;58:1-25.
31. Ram A. No clinical trials carried out, TN, Kerala
to introduce controversial vaccine. Available from:
http://epaper.timesofindia.com/Repository/ml.asp?Ref=VE9JQ0gvMjAxMS8xMC8xOSNBcjAwMz
A2&Mode=HTML&Locale=english-skin-custom Accessed on December 14, 2011.
32. Tripathi A. Petition against pentavelent vaccine.
Available from: http://ibnlive.in.com/news/petition-against-pentavelent-vaccine/211694-17.html
Accessed on December 14, 2011.
33. Rudan I, Boschi-Pinto C, Biloglav Z, Mulholland
K, Campbell H. Epidemiology and etiology of childhood pneumonia. Bull
World Health Organ. 2008;86:408-16.
34. Gupta M, Kumar R, Deb AK, Bhattacharya SK, Bose
A, John J, et al. Multi-center surveillance for pneumonia &
meningitis among children (<2 yr) for Hib vaccine probe trial
preparation in India. Indian J Med Res. 2010;131:649-65.
35. Broor S, Parveen S, Bharaj P, Prasad VS,
Srinivasulu KN, Sumanth KM, et al. A prospective three-year
cohort study of the epidemiology and virology of acute respiratory
infections of children in rural India. PLoS One. 2007;2:e491.
36. Johnson HL, Kahn GD, Anne M, Palaia AM, Levine
OS, Santosham M. Comprehensive review characterizing the epidemiology of
streptococcus pneumoniae in India. The 8th International Symposium on
Pneumococci and Pneumococcal Diseases (ISPPD). Iguaçu Falls, Brazil,
11-15 March 2012, Abstract # A-428-0023-00743.
37. Mathew JL, Patwari AK, Gupta P, Shah D, Gera T,
Gogia S, et al. Acute respiratory infection and pneumonia in
India: a systematic review of literature for advocacy and action:
UNICEF-PHFI series on newborn and child health, India. Indian Pediatr.
2011;48:191-218.
38. Johnson HL, Bassani DG, Perin J, Levine OS,
Cherian T, O’Brien KL. Burden of childhood mortality caused by
streptococcus pneumoniae in India. The 8th International Symposium
on Pneumococci and Pneumococcal Diseases (ISPPD). Iguaçu Falls, Brazil,
11-15 March 2012, Abstract # A-428-0023-00743.
39. Nisarga RG, Balter I, Premalatha R, Govindaraj M,
Batuwanthudawe R, Moscariello M, et al. Prospective,
multinational, active, hospital-based epidemiologic surveillance for IPD
and pneumonia burden among children in Bangalore south-zone, Bangalore,
India. Presented at the 29th Annual Meeting of the European Society for
Paediatric Infectious Diseases (ESPID) June 2011. The Hague,
Netherlands.
40. Minz S, Balraj V, Lalitha MK, Murali N, Cherian
T, Manoharan G, et al. Incidence of Haemophilus influenzae type b
meningitis in India. Indian J Med Res. 2008;128:57-64.
41. John TJ, Pai R, Lalitha MK, Jesudason MV,
Brahmadathan KN, Sridharan G, et al. Prevalence of pneumococcal
serotypes in invasive diseases in southern India. Indian J Med
Res.1996;104:205-7.
42. Invasive Bacterial Infections Surveillance (IBIS)
Group, International Clinical Epidemiology Network (INCLEN). Prospective
multicentre hospital surveillance of Streptococcus pneumoniae disease in
India. Lancet.1999;353:1216-21.
43. Kanungo R, Rajalakshmi B. Serotype distribution &
antimicrobial resistance in Streptococcus pneumonia causing invasive &
other infections in south India. Indian J Med Res. 2001;114:127-32.
44. Levine OS, Cherian T, Hajjeh R, Knoll MD.
Progress and future challenges in coordinated surveillance and detection
of pneumococcal and Hib disease in developing countries. Clin Infect
Dis. 2009;48:S33-6.
45. Shin J, Baek JY, Kim SH, Song JH, Ko KS.
Predominance of ST320 among Streptococcus pneumoniae serotype 19A
isolates from 10 Asian countries. J Antimicrob Chemother.
2011;66:1001-4.
46. Kim SH, Song JH, Chung DR, Thamlikitkul V, Yang
Y, Wang H, et al. Changing trend of antimicrobial resistance and
serotypes in Streptococcus pneumoniae in Asian countries: an
ANSORP study. Antimicrob Agents Chemother. 2012;56:1418-26.
47. Bravo LC. Asian Strategic Alliance for
Pneumococcal Disease Prevention (ASAP) Working Group. Overview of the
disease burden of invasive pneumococcal disease in Asia. Vaccine.
2009;27:7282-91.
48. Vashishtha VM, Kumar P, Mittal A. Sero-epidemiology
of Streptococcal Pneumoniae in developing countries and issues
related to vaccination. Journal of Pediatric Sciences. 2010;5:e48.
49. Johnson HL, Deloria-Knoll M, Levine OS, Stoszek
SK, Freimanis Hance L, Reithinger R, et al. Systematic evaluation
of serotypes causing invasive pneumococcal disease among childrenunder
five: the pneumococcal global serotype project. PLoS Med. 2010;7.
pii:e1000348.
50. Selva L, Ciruela P, Esteva C, de Sevilla MF,
Codina G, Hernandez S, et al. Serotype 3 is a common serotype
causing invasive pneumococcal disease in children less than 5 years old,
as identified by real-time PCR. Eur J Clin Microbiol Infect Dis. 2011
Nov 4. [Epub ahead of print]
51. Saha SK, Rikitomi N, Biswas D, Watanabe K,
Ruhulamin M, Ahmed K, et al. Serotypes of Streptococcus
pneu-moniae causing invasive childhood infections in Bangla-desh, 1992
to 1995. J Clin Microbiol. 1997;35:785-7.
52. Arifeen SE, Saha SK, Rahman S, Rahman KM, Rahman
SM, Bari S, et al. Invasive pneumococcal disease among children
in rural Bangladesh: results from a population-based surveillance. Clin
Infect Dis. 2009;48:S103-13.
53. Saha SK, Naheed A, El Arifeen S, Islam M, Al-Emran
H, Amin R, et al. Surveillance for invasive Streptococcus
pneumoniae disease among hospitalized children inBangladesh:
antimicrobial susceptibility and serotype distribution. Clin Infect Dis.
2009;48:S75-81.
54. Paradiso P, Rodgers GL, Girgenti D, et al.
Inducing Protective Immunity to Pneumococcal Serotype 3: Impact of
Schedule, Geography, and Immune Parameters on the Response to
CRM197-Based PCV13. Presented at the 7th International Symposium on
Pneumococci and Pneumococcal Diseases, March 14–18, 2010, Tel Aviv,
Israel.
55. Vesikari T, Wysocki J, Chevallier B, Karvonen A,
Czajka H, Arsène JP, et al. Immunogenicity of the 10-valent
pneumococcal non-typeable Haemophilus influenzae protein D conjugate
vaccine (PHiD-CV) compared to the licensed 7vCRM vaccine. Pediatr Infect
Dis J. 2009;28:S66-76.
56. Department of Health and Human Services. Clinical
Review of Biologics License Application for Prevnar 13 (Pneumococcal
13-valent Conjugate Vaccine (Diphtheria CRM197 Protein)). 2010.
Available from:
http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM206341.pdf.
Accessed on March 1, 2012.
57. Tregnaghi MW, Sáez-Llorens X, López P, Abate H,
Smith E, Pósleman A, et al. Evaluating the efficacy of 10-valent
pneumococcal non-typeable Haemophilus influenzae protein-D conjugate
vaccine (PHiD-CV) against community-acquired pneumonia in Latin America.
The 29th Annual Meeting of the European Society for Paediatric
Infectious Diseases (ESPID). The Hague, The Netherlands; 7-11 June 2011.
Abstract 1411.
58. Andrade AL, Ternes YM, VieiraMA, Sgambatti S,
Lamaro-Cardoso5 J, Kipnis A, et al. Effectiveness of 10-valent
conjugate pneumococcal vaccine on community-acquired pneumonia and
pneumococcal carriage after vaccination in Brazil: A population-based
study. Presented at the 7th World Congress of the World Society for
Pediatric Infectious Diseases (WSPID), Melbourne, Australia, 16-19
November 2011, Abstract 670.
59. Miller E, Andrews NJ, Waight PA, Slack MP, George
RC. Effectiveness of the new serotypes in the 13-valent pneumococcal
conjugate vaccine. Vaccine. 2011;29:9127-31.
60. Poolman J, Frasch C, Nurkka A, Käyhty H, Biemans
R, Schuerman L. Impact of the conjugation method on the immunogenicity
of Streptococcus pneumoniae serotype 19F polysaccharide in conjugate
vaccines. Clin Vaccine Immunol. 2011;18:327-36.
61. Hausdorff WP, Hoet B, Schuerman L. Do
pneumococcal conjugate vaccines provide any cross-protection against
serotype 19A? BMC Pediatr. 2010 Feb 2;10:4.
62. Shinefield H, Black S, Ray P, Fireman B, Schwalbe
J, Lewis E. Efficacy, immunogenicity and safety of heptavalent
pneumococcal conjugate vaccine in low birth weight and preterm infants.
Pediatr Infect Dis J. 2002;21:182-6.
63. John TJ, Vashishtha VM. Path to polio eradication
in India: A major milestone. Indian Pediatr. 2012;49:95-8.
64. World Health Organization. A New Strategy for the
‘Polio Endgame’? Available from:
http://www.who.int/immunization_standards/vaccine_quality/new_strategy_polio.pdf
Accessed on March 3, 2012.
65. Cáceres VM, Sutter RW. Sabin monovalent oral
polio vaccines: review of past experiences and their potential use after
polio eradication. Clin Infect Dis. 2001;33:531-41.
66. Dömök I. Experiences associated with the use of
live poliovirus vaccine in Hungary, 1959–1982. Rev Infect Dis.
1984;6:S413–S8.
67. Von Magnus H, Petersen I. Vaccination with
inactivated poliovirus vaccine and oral poliovirus vaccine in Denmark.
Rev Infect Dis. 1984;6:S471-4.
68. Modlin JF, Halsey NA, Thoms ML, Meschievitz CK,
Patriarca PA. Humoral and mucosal immunity in infants induced by three
sequential inactivated poliovirus vaccine-live attenuated oral
poliovirus vaccine immunization schedules. Baltimore Area Polio Vaccine
Study Group. J Infect Dis. 1997;175:S228-34.
69. World Health Organization. Polio vaccines.
Grading tables. Table V: Sequential administration IPV-OPV. Available
from:
http://www.who.int/entity/immunization/polio_sequential_administration_IPV_OPV.pdf
Accessed on March 4, 2012.
70. Polio vaccines and polio immunization in the
pre-eradication era: WHO position paper. Wkly Epidemiol Rec.
2010;85:213-28.
71. Recommended Childhood and Adolescent Immunization
Schedules-United States, 2012, Committee on Infectious Diseases.
Pediatrics 2012;129;385 Available from:
http://pediatrics.aappublications.org/content/129/2/385.full.html
Accessed on March 4, 2012.
72. Recommended composition of influenza virus
vaccines for use in the 2012-2013 northern hemisphere influenza seasons.
February 2012. Available from:
http://www.who.int/influenza/vaccines/virus/recommendations/201202_recommendation.pdf
Accessed on March 4, 2012.
73. Recommended composition of influenza virus
vaccines for use in the 2012 southern hemisphere influenza season
September 2011. Available from:
http://www.who.int/influenza/vaccines/virus/recommendations/2011_09_recommendation.pdf
Accessed on March 4, 2012.
74. Chadha MS, Broor S, Gunasekaran P, Potdar VA,
Krishnan A, Chawla-Sarkar M, et al. Multisite virological
influenza surveillance in India: 2004-2008. Influenza Other Respi
Viruses. 2011 Sep 29. doi: 10.1111/j.1750-2659.2011.00293.x. [Epub ahead
of print]
|

