Fibrous dysplasia (FD) is a rare disorder wherein scar tissue replaces
normal bone-tissue, weakens the bone, causing deformity and intense pain.
We present 2 cases of FD due to McCune Albright Syndrome (MAS) showing
remarkable clinical improvement with pamidronate.
An 8 year-old girl presented with excessive weight gain
since 3 years after fracture of right tibia/fibula following a trivial
trauma; early fatigue, generalized bone pains and inability to bear weight
due to extreme right leg pain. She weighed 45 kg with body mass index of
26.6 kg/m2. Her height was 118 cm. She had multiple
café-au-lait spots and bilateral genu valgum. Her pubertal status was
B2P1A1M0, indicating early puberty. Serum calcium (9.6 mg/dL), phosphorus
(4.8 mg/dL), parathyroid hormone (42 pg/mL, normal range: 9-65 pg/mL) and
25-hydroxyvitamin D (25OHD3) (21 ng/mL, normal range: 12-40 ng/mL) were
normal, while alkaline phosphatase (ALP) (609 IU/L) (range: 40-240 IU/L)
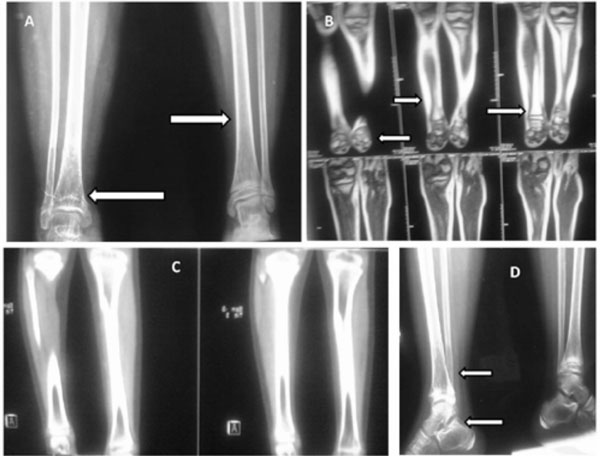
was high. Skeletal survey showed healing fracture of tibia with distal
tibial cystic lesion, generalized osteopenia of foot bones with cortical
thinning of leg bones (Fig.1). Radiograph of shoulders
revealed patchy sclerotic areas in proximal ends of both humerii with
‘cotton wool’ appearance. The diagnosis of FD was confirmed by MRI of
right ankle and Dexa and bone scan (Fig.1).
 |
|
Fig.1 A: X ray of bilateral tibia and
fibula with ankles depicting healing fracture of tibia with distal-tibial
cystic lesion with hohmogeneous ground glass matrix and cloud of
smoke calcification, generalized osteopenia of foot bones with
cortical thinning of leg bones. B: T2 weighted images of MRI
depicting heterogenous hyperintensity in distal third of right tibia
and small heterogeous hyperintensities in ankle bones. C: A section
from computed tomography scan showing ground glass appearance of
lower end of tibia. D: Lateral X ray depicting distal tibial cystic
lesion, healing fracture and osteopenia of foot bones especially
talus and calcaneus. |
The second case was a 2 year-old female child with
complaints of limping, bony pains, flat foot, and deformity of right ankle
since 1 and a half years of age, which gradually progressed to right leg
lengthening at presentation to our institute. She weighed 16.6 kg while
her length was 82.3 cm. She had multiple café-au-lait spots (>6 mm with
irregular borders). Parathyroid hormone levels were 19.6 pg/mL (normal
range: 9-65 pg/mL). ALP was 585 IU/L (normal range: 40-240 IU/L) while
calcium and phosphorus were 8.5 mg/dL and 4.1 mg/dL, respectively. Nuclear
scan showed mildly increased tracer distribution in right tibia and left
fibula, suspicious of FD. MRI right leg showed remodeling of proximal
third of right tibia with cortical/periosteal thickening and depressed
postero-medial bone with focal cortical break in proximal third with
surrounding tissue edema. The left fibula also showed remodeling along
with periosteal thickening in the middle third.
Both patients were diagnosed as MAS and treated with
pamidronate (in view of pain and reduced mobility) 1 mg/kg/day for 3 days
given 3 monthly for 6 cycles along with metformin, calcium and vitamin D
supplementation with appropriate diet, whereafter they improved. During
the administration of pamidronate patients were under continuous
electrocardiogram monitoring while vital signs were frequently recorded.
Hypocalcemia (biochemical and manifest) was specifically observed.
Calcium, phosphorus, ALP, 25OHD3 levels (27.5 ng/mL in case 1 and 32.4 ng/mL
in case; normal range: 12-40 ng/mL) were normal at follow-up.
At 2 years follow-up, they showed improvement in pain
(assessed by visual analogue scale), mobility, deformity, general
well-being and quality of life (ALP improved after 1 year). These 2
patients were able to perform all the activities of daily living
appropriate to their age whilst the parents were satisfied with their
overall progress. To the best of our knowledge, this is the first report
from India describing role of pamidronate in FD due to MAS.
FD is characterized by replacement of normal bone
tissue by fibrous connective tissue with a characteristic whorled pattern
containing trabeculae of immature non-lamellar bone. Histopathologically,
FD shows fibrous stroma with spicules of disconnected woven bone with a
few mature osteoblasts and osteoclasts. Biphosphonates inhibit osteoclasts,
reduce bone resorption and can lead to refilling of dysplastic lesions.
As observed by most other investigators, our
observations highlight that good results can be obtained with pamidronate
in FD, which should be administered early to halt disease progression,
preserve bone mass, reduce fracture rates, avoid deformities, alleviate
symptoms and delay/avoid surgery(1,2). Since standard guidelines for its
use are unavailable, therapeutic response to pamidronate is noteworthy
while longterm follow-up is awaited(3). Pamidronate therapy appears to be
useful in children and adolescents with FD with a good short term safety
profile. Potential multisystem (renal, hepatic, cardiovascular,
gastrointestinal and skeletal) and oncological adverse effects of long
term use are open to observation and speculation(4,5).
References
1. Lala R, Matarazzo P, Andreo M, Marzari D, Bellone J,
Corrias A, et al. Bisphosphonate treatment of bone fibrous
dysplasia in McCune-Albright syndrome. J Pediatr Endocrinol Metab 2006;
19: 583-593.
2. Chan B, Zacharin M. Pamidronate treatment of
polyostotic fibrous dysplasia: failure to prevent expansion of dysplastic
lesions during childhood. 2006; 19: 75-80.
3. Chapurlat RD, Orcel P. Fibrous dysplasia of bone and
McCune-Albright syndrome. Best Pract Res Clin Rheumatol 2008; 22: 55-69.
4. Zacharin M, O’Sullivan M. Intravenous pamidronate
treatment of polyostotic fibrous dysplasia associated with the McCune
Albright syndrome. J Pediatr 2000; 137: 403-409.
5. Papapetrou PD. Bisphosphonates-associated adverse events. Hormones
2009; 8: 96: 110.

