|
K.P. Sanghvi, R.H. Merchant, Alka
Karnik and Aparna Kulkarni
From the Nowrosjee Wadia Maternity Hospital, Acharya Donde
Marg, Parel, Mumbai 400012, India
Reprint requests: Dr. R.ll. Merchant, Director - Neonatal
Services, Nowrosjee Wadia Maternity Hospital, Acharya Donde Marg, Parel,
Mumbai 400 012, India
Manuscript received: July 29, 1998; Initial review completed:
September 10, 1998; Revision accepted: January 6, 1999.
Objective: To determine the role of ethamsylate in prevention of
PVH-IVH in premature infant.v <34 weeks gestational age. Design:
Prospective, randomized, controlled study. Methods: Infants less than
34 weeks gestational age were included in the trial. Neonates
with congenital malformations, family history of bleeding disorders and
with Apgar scores <5 at 5 minutes were excluded. Subjects
were randomized into two groups - Group A infants received
intravenous ethamsylate (12.5 mg/kg) six hourly forfour days and Group B
infants served as a control group. Regular cranial ultrasounds to detect
the presence of PVH-IVH were done between days 3-5, 1014 and
28-30 of post natal age, and before hospital discharge in all infants
and weekly in infants detected to have PVH-IVH on earlier scans. Various
antenatal and postnatal factors known to affect the incidence of PVH-IVH
were recorded. Results: A total of 192 infants underwent the
trial, 93 in Group A and 99 in Group B. Antenatal
corticosteroids (lor 2 doses) were administered to 32 (
34.4%) and 36 (36.3%) women in Group A and Group B,
respectively. None of the mothers received phenobarbitone, vitamin K or
indomethacin antenatally and none of the infants received phenobarbitone,
vitamin E or indomethacin postnatally during the study period. PVH-IVH
was seen in 26 infants in Group A, of which Grade IIVH occurred
in 9, Grade 11 in 14, Grade 111 in 2 and Grade IV in
one infant. Twenty-nine infants had PVH-IVH in Group B of which I I
had Grade I, 15 Grade 11 and 3 Grade Ill. None of the differences
were statistically signifcant. Conclusion: Postnatal administration of
ethamsylate did /'lot decrease the incidence of PVH-IVH in the study
infants.
Key words: Ethamsylate, Periventricular-intraventricular
hemorrhage, Premature infants
Periventricular intraventricular hemorrhage (PVH-IVH) has been
documented to occur in 40% of very low birth weight infants (VLBW)(l).
The neurodevelopmental sequelae of PVH-IVH remain a major cause for
concern. Approximately half of PVH-IVH occur within the first day of
life an 90%
occur before 4 days of age. Further progression in the size of the
hemorrhage may be found in 20-40% of the infants in the first week of
life(2,3).
The efficacy of
antenatal and postnatal interventions in the form of drugs like
phenobarbitone(4-6), vitamin E(7), indome thacin(8), ethamsylate(9-12)
and vitamin K(13) for prevention of PVH-IVH have been studied. However,
no consensus exists regarding the value of drug prophylaxis for
prevention of PVH-IVH.
Ethamsylate (Diethyl ammonium 2.5- dihydroxybenzene sulphonate) is a
capillary stabilizing agent and an inhibitor of prostaglandin synthesis.
It lowers Prostaglandin 12 and vasoconstrictor thromboxane levels,
thereby reducing baseline cerebral blood flow(14). This effect may in
part, explain the means by which it can prevent PVH-IVH in preterm
infants. Ethamsylate is a safe cheap and an easily available drug. There
have
been few studies that
have
defined the role of this drug in the prevention
ofPVH-IVH(9-12). The present study was carried out to evaluate the
efficacy of ethamsylate in preventing PVH-IVH in preterm infants <34
weeks gestation age, in an attempt to find a safer and cheaper
alternative to the other known drugs.
Subjects and Methods
Study Group
This randomized, controlled trial was carried out on infants <34 weeks
gestational age, which was determined by the best obstetrical estimate.
Inborn infants between 1st December 1996 and 28th February 1998 were
included in the trial. Newborns with congenital malformations, family
history of bleeding disorders and with Apgar scores <5 at 5 minutes were
excluded from the trial. Infants who did not complete the drug schedule
were also excluded. Each eligible neonate was randomized into 'ethamsylate'
[Group A] or 'control' [Group B] groups using opaque envelopes.
Administration of the Study Drug
The infants in Group A received 12.5 mg! kg (0.1 ml/kg) of ethamsylate (Ethamsyl
-
Mejda Pharmaceuticals) intravenously within the first hour of birth and
thereafter 6 hourly for 4 days. The Group B infants did not receive any
medication other than routine therapy.
Evaluation of Infants
Cranial ultrasounds were obtained on all study infants by a pediatric
sonologist, who was blinded to the patient group, on days 3-5, 10-14 and
28-30 of post natal age and before discharge using a WIPRO Scanner 2000
ultra- sound real time machine with 7.5 and 5 Hz probes. Scans was
performed weekly if PVH- IVH or its complications were found on initial
scans. Ultrasound appearances of PVH-IVH were graded from I-IV by a
system based on that of Papi1e et al.(1).
Clinical data relating to factors known to be associated with an
increased incidence of peri ventricular-intra ventricular hemorrhage (PVH-IVH)
were noted. Antenatal history of pregnancy induced hypertension, other
maternal medical disorders, drug administration (steroids,
phenobarbitone, vitamin K, indomethacin), delivery details including
fetal distress, instrumentation and Apgar scores, and the clinical
course of the infants with specific regards to ventilation, surfactant
therapy, PDA, sepsis and medications (phenobarbitone, vitamin E,
indomethacin) administered were noted. Side effects of ethamsylate
i.e., skin rashes, hypotension, and deep vein thrombosis were
recorded. Other known adverse reactions of ethamsylate such as nausea
and headaches could not be noted due to the age constraints of the
patient group.
Outcome Measures
The outcome mesures were the incidence and severity of PVH-IVH during
the neonatal period in the two groups during the neonatal period.
Statistical Analysis
Statistical analysis was done using the Student's t-test for continuous
variables and the Chi-square test for discrete variables. A p value
<0.05 was taken as statistically significant.
Results
A total of 208 infants < 34 weeks delivered at the hospital during the
study period met the inclusion criteria. Of these four infants with
congenital heart disease, two infants with geni- tourinary tract anomaly
and two with intestinal obstruction were excluded after randomization.
Eight infants died before completing the drug schedule, five due to
congenital sepsis and three due to respiratory distress syndrome. Of the
remaining 192 infants, 93 belonged to Group A and 99 to Group B.
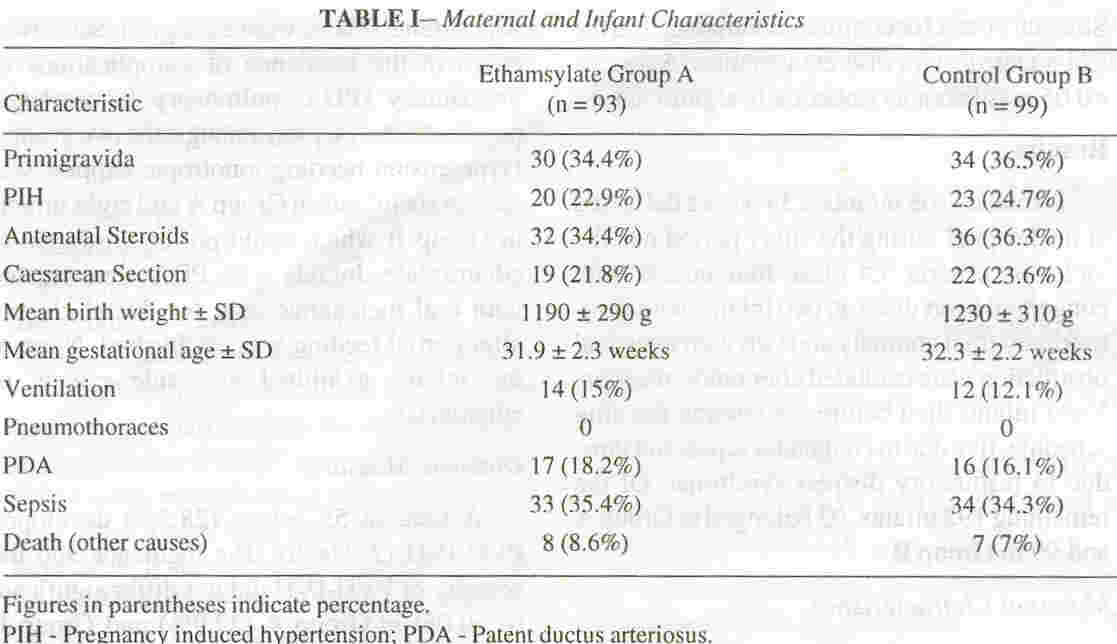
Maternal Characteristics
The frequency of antenatal administration of corticosteroids and the
mode of delivery were not found to be statistically different amongst
the two groups (Table /). None of the mothers received
phenobarbitone, vitamin K or indomethacin antenatally. There were two
twins and one set of triplets in Group A, as com- pared to three twin
pairs in Group B. Five twin pairs were split with one infant in Group A
and the other in Group B.
Characteristics of Infants
The mean birth weights and gestational ages were 1190:!:
290 grams (range 760-1600 grams) and 31.9:!: 2.3 weeks (range 26-34
weeks) in Group A and 1230:!: 310 grams
(range 820-1740 grams) and 32.3
:!:
2.2 weeks (range 26-34 weeks) in Group B, respectively (Table I).
In Group A and Group B, 76.3% and 77 .8% of the infants respectively
were greater than 28 weeks of gestation. No infant received
phenobarbitone, or indomethacin during the first seven days or
prophylactic Vitamin E for prevention of retinopathy of prematurity
during the study period. The frequency of respiratory failure requiring
ventilation in the two groups was similar. There were no significant
differences in the incidence of complications of prematurity (PDA,
pulmonary hemorrhage, pneumothorax, sepsis) amongst the two groups.
Hypotension needing ionotropic support was seen in six infants in Group
A and eight infants in Group B which could not be attributed to
ethamsylate. Infants with PDA were treated with oral mefenamic acid for
ductal closure after partial feeding was established. None of the
infants exhibited any side effects of ethamsylate.
Outcome Measures
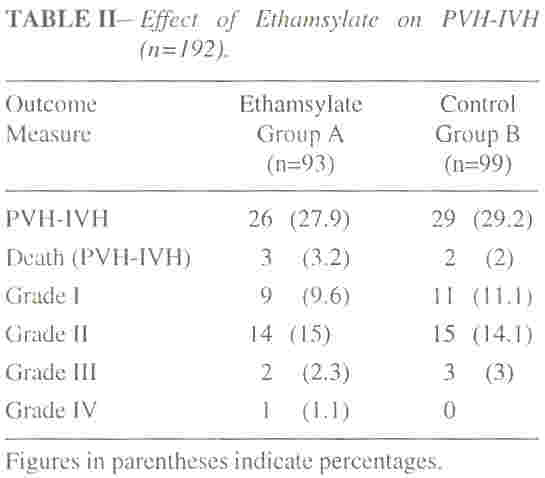
A total of 55 babies (28.5%) developed PVH-IVH (Table II). The
incidence and the severity of PVH-IVH did not differ significant (p
>0.05) in Group A (27.9%) and Group B (29.2%) though the babies in Group
B had a higher mean birth weight and gestational age. Periventricular
leukomalacia and ventriculitis with progressive hydrocephalus were
detected in one infant each in group B.
Mortality
Eleven infants in Group A and nine infants in Group B died in hospital.
In Group A causes of mortality were necrotising enterocolitis - three,
sepsis-three, PVH-IVH- three, RDS- one, and pulmonary hemorrhage- one.
Group B had nine deaths which were inclusive of six due to sepsis, two
due to PVH- IVH and one due to pulmonary hemorrhage.
Discussion
Four studies relating to ethamsylate prophylaxis for PVH-IVH have been
published(9-12). One study(9) enrolled 70 infants in a randomized
double-blind trial and demonstrated
a
significant reduction (50%) in the frequency of PVH-IVH. Forty-five
ventilated infants given ethamsylate in another study were found to have
a reduction in both the incidence and severity of PVH-IVH as compared to
controls(10). Two large randomized controlled multi-centric trials(11-12) were conducted to evaluate the role of ethamsylate in the prevention of PVH-IVH. The study by Benson et al. (1 J) demonstrated a 50% reduction in major bleeds whereas the EC ethamsylate trial group( 12) found no differences either in the incidence or in the severity of PVH-IVH with the use of postnatal prophylactic ethamsylate.
The duration and dosage of ethamsylate administered to the newborns in our study was similar to other studies(9-12). The mean birth weights of the infants enrolled in these studies was comparable but the mean gestational age was higher in our study as more than 80% of the infants were >28 weeks gestation. The incidence of PVH-IVH
decreases with increasing gestational age and weight(1). The overall incidence of PVH-IVH in this study was lower than 25-45% reported by other studies on ethamsylate(9,1 J, 12). Severe grades were seen in one-fifth of the infants who developed PVH- IVH in our study as compared to almost one- third of the infants in the previous trials(9- 12).The incidence of RDS, ventilation, pneumothoraces and clinical PDA in babies <34 weeks gestation (Table I) at our institute was low as compared to the two multicentric trials(ll, 12) which have reported an incidence of RDS (68%), ventilation (70-80%), pneumo- thoraces (15%) and PDA (20%) all of which are known to increase the risk of PVH-IVH. Antenatal steroids were administered
to 35% of
the babies in our trial as compared to 16% in the EC ethamsylate trial group study(12). The other studies(9-ll) have not reported data regard- ing antenatal steroid use or any other drug administered for prevention of PVH-IVH. Prenatal steroids are known to decrease the incidence of PVH-IVH(15). Prophylactic vitamin E, phenobarbitone, and indomethacin
were not received by any of the mothers or infants in this study. Postnatal vitamin E was not found to affect the incidence or severity of PVH-IVH(lI, 12), and as a unit policy we do not use prophylactic vitamin E for prevention of retinopathy of prematurity except for multi- vitamin supplementation after the infant is on full oral feeds. The combined effect of higher coverage of mothers in preterm labor with antenatal steroids, the lower incidence of RDS and ventilation and its complications, and the higher gestational age of our study infants may have resulted in the decreased- incidence and severity of PVH-IVH in the present study.
We did not include newborns with low Apgar scores to avoid a confounding factor at study entry. This also precluded the use of phenobarbitone in asphyxiated infants with convulsions which is known to affect PVH- IVH(6). Hospital records of the previous' two years showed that PVH-IVH occurred in 30.8% of infants <34 weeks gestation. Power calculations suggested that to attain a statistical significance level of p <0.05 with a power of 80%, a total number of 174 infants would be required in each group to decrease the incidence of PVH-IVH to 15% with prophylactic ethamsylate. A review was requested by the sponsors midway through the study and when no obvious advantage was found the study was stopped with mutual consent.
In conclusion, this study could not conclusively prove or disprove the efficacy of ethamsylate in prevention of PVH-IVH as the incidence of severe grades of PVH-IVH was
low in the study infants. We feel that °v:erall improvements in care in the perinatal and early neonatal period, including antenatal cortico steroid therapy, preventing RDS
and the complications of ventilation may contribute more to decrease the severity of PVH-IVH rather than postnatal ethamsylate therapy though it was not the subject of comparison in our study.
|
1.
Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: A study of infants with birth weight less than 1500 grams. J Pediatr 1978; 92: 529-534.
2.
De Crespigny LC, Mackay R, Murton LJ. Tim- ing of neonatal cerebroventricular hemorrhages with ultrasound. Arch Dis Child 1982; 57: 231- 234.
3.
Dolfin T, Skidmore MB, Fong FW. Incidence, severity and timing of subependymal and intra- ventricular hemorrhages in preterm infants born in a perinatal unit as detected by serial real time ultrasound. Pediatrics 1983; 71: 541-546.
4.
Donn SM, Roloff OW, Goldstern GW. Preven- tion of IVH in preterm infatns by phenobarbitone: A controlled trial. Lancet 1981; i i: 215-217.
5.
Bedard MP, Shankaran S, Solvis TL, Pantojo A, Dayal B, P?land RL. Effect of prophylactic phenobarbitill in IVH in high risk infants. Pedi- atrics 1984;73: 435-439.
6.
Shankaran S, Papile L, Wright LL, Ehrenkranz RA, Mele L, Lemons JA, et al. The effect of antenatal phenobarbital on neonatal intracranial hemorrhage in preterm infants. N Eng J Med 1997; 337; 466-471.
7.
Chiswick ML, Johnson M, Woodhall C. Protec- tive effect of Vitamin E against IVH in prema- ture babies. Br Med J 1983; 287: 81-84. --
8.
Bada HS, Green RS, Pourcyrous M. Indomethacin reduces the risks of severe IVH. J Pediatr 1989; 115: 631-637.
9.
Morgan ME!, Benson JWT, Cooke RWI. Ethamsylate reduces the incidence of PVH in VLBW babies. Lancet 1981; 2: 830-831.
10.
Cooke RWI, Morgan MEI. Prophylactic ethamsylate for PVH. Arch Dis Child 1984; 59: 82-83.
11.
Benson JWT, Drayton MR, Hayward C. Multicenter trial of ethamsylate for prevention of PVH in VLBW infants. Lancet 1986; 2: 1297- 1300.
12.
The EC Ethamsylate Trial Group. The EC ran- domized controlled trial of prophylactic ethamsylate for very preterm neonates: Early mortality and morbidity. Arch Dis Child 1994; 70: F201-F205.
13.
Kazzi NJ, Ligan NB, Liang KC. Maternal ad- ministration of Vitamin K does not improve coagulation profile of preterm infants. Pediatrics 1989; 84: 1045-1050.
14.
Ment LR, Stewart WB, Duncan Cc. Beagle puppy model of IVH: Ethamsylate studies. Pros- taglandins 1984; 27: 245-256.
15.
Effect of corticosteroid for fetal maturation on perinatal outcomes. NIH Consensus Statement, Vol 12, No 2. Bethsda, NIH Office of Medical Applications of Research, 1994; pp 1-24.
|
