|
|
|
Indian Pediatr 2021;58:
80-81 |
 |
Wolcott-Rallison Syndrome Affecting Three
Consecutive Conceptions of a Consanguineous Couple
|
|
Rajendra Prasad Anne,1* Madhavi Vasikarla2
and Tejo Pratap Oleti1
Department of 1Neonatology and 2Genetics,
Fernandez Foundation, Hyderabad, Andhra Pradesh, India.
Email:
[email protected]
|
|
Permanent neonatal diabetes mellitus (NDM) is a debilitating condition.
In couples with consanguineous marriage, and especially with multiple
children being affected, a strong possibility of genetic causes should
be kept and evaluated appropiately. Wolcott-Rallison syndrome is one
such syndrome, now being more commonly diagnosed in Indian families. A
couple presented to the fetal medicine unit for genetic counselling at a
gestational age of 9 weeks, because of two previous babies being
affected with early onset type 1 diabetes mellitus. There was a 3rd
degree consanguinity. The elder child was 6 year-8 month-old girl, with
hyperglycemia detected at 1 month of age. She was evaluated by the
pediatrician. She had history of multiple episodes of seizures,
unconsciousness and developmental delay in all fields, with a motor age
of 2 years and social and language age of 4 years. Parents were not
compliant with either regular insulin administration or home blood
glucose monitoring. On examination, she was stunted (height 86 cm, <3rd
centile), underweight (weight 11.4 kg, <3rd centile) and had
microcephaly (head circumference 43 cm, <3rd centile). The child had a
chubby look, round facies, delayed dentition, dental caries and short
stubby fingers. There was firm hepatomegaly with liver span of 12 cm. We
evaluated for hypothyroidism, celiac disease, polyendocrinopathies and
genetic syndromes causing type 1 diabetes mellitus. There were no
clinical features suggestive of exocrine pancreatic insufficiency. The
younger sibling was a female child who was diagnosed as type 1 diabetes
mellitus at 1 month of life and succumbed the following month. Medical
records were unavailable. A detailed three-generation pedigree did not
reveal any other family member with similar manifestations.
The skeletal survey revealed a bone age >4 years,
with small carpals, hypo-mineralized metacarpals, and notching of
anterior vertebrae, suggestive of skeletal dysplasia (Fig. 1).
The laboratory evaluation did not reveal any abnormalities in hemoglobin,
leucocyte counts, thyroid function tests and liver enzymes. Blood urea
nitrogen was 15.3 mmol/L, creatinine was 61.9 µmol/L, and glycosylated
hemoglobin was 12.7%. A genetic panel for causes of PNDM and
maturity-onset diabetes of the young (MODY) was done. It revealed a
homozygous non-sense variation in exon 17 of the EIF2AK3 gene
(chr2:g. 88857412G>A), consistent with Wolcott-Rallison syndrome.
Subsequently, we carried out sanger variant analysis for the same gene
in the fetus by chorionic villus sampling at 14 weeks of gestation. It
also revealed homozygosity for chr2:g. 88857412G>A and c.3193C>T. The
couple were advised termination of pregnancy and were counseled
regarding recurrence risk and need for antenatal diagnosis. The
importance of home blood glucose monitoring and insulin administration
was explained for the older child and management plan with concerned
specialist was arranged.
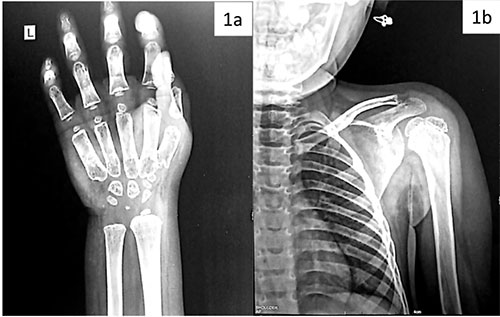 |
|
Fig. 1 (a) X-ray wrist anteroposterior view of the
index child, and (b) X-ray showing thoracic spine, ribs
and humerus.
|
Neonatal diabetes mellitus is a rare form of type 1
diabetes mellitus, with onset in first 6 months of life and an incidence
of 1 in 90,000 to 1,60,000 live births [1]. Most NDMs are monogenic, and
can be either transient or permanent. The most common mutations causing
NDM worldwide are related to defects in potassium channel subunit genes,
namely KCNJ11 and ABCC8 [2]. Similarly, in India, most
published literature shows that the commonest mutations are related to
potassium channel mutations [3]. In the setting of parental
consanguinity, most common causes related to an autosomal recessive
inheritance. These include mutations in EIF2AK3, GCK, GLIS3, RFX6,
IER31P1 and MNX1 genes. Except GCK1, the remaining mentioned
mutations result in syndromic forms of type 1 diabetes mellitus, with
extra-pancreatic involvement [1,4]. Thus, it becomes important to get
focused evaluation for autosomal recessive conditions in such a
scenario. Wolcott-Rallison syndrome is being recognized as an important
cause of syndromic permanent NDM in Indian subcontinent [5,6]. This
syndrome has high mortality and several associated morbidities including
skeletal dysplasia, episodic liver failure, renal dysfunction, exocrine
pancreas insufficiency and developmental delay. The frequency of
extra-pancreatic manifestations increases with increasing age, with
initial appearance of skeletal abnormalities, followed by liver and
renal dysfunction. In the index child, we could note developmental delay
and skeletal dysplasia.
Evaluation for monogenic causes should be done in all
cases of permanent NDM. When associated with consanguinity and
extra-pancreatic manifestations, syndromic neonatal diabetes mellitus
with autosomal recessive inheritence is most likely. Timely genetic
diagnosis and prenatal confirmation can avert birth of an affected
progeny.
REFERENCES
1. Lemelman MB, Letourneau L, Greeley SAW. Neonatal
diabetes mellitus: An update on diagnosis and management. Clin Perinatol.
2018;45:41-59.
2. De Franco E, Flanagan SE, Houghton JAL, et al. The
effect of early, comprehensive genomic testing on clinical care in
neonatal diabetes: an international cohort study. Lancet.
2015;386:957-63.
3. Jain V, Satapathy A, Yadav J, et al. Clinical and
molecular characterization of children with neonatal diabetes mellitus
at a tertiary care center in northern India. Indian Pediatr.
2017;54:467-71.
4. Flannick J, Johansson S, Njølstad PR. Common and
rare forms of diabetes mellitus: towards a continuum of diabetes
subtypes. Nat Rev Endocrinol. 2016;12:394-406.
5. Jahnavi S, Poovazhagi V, Kanthimathi S, Gayathri V, Mohan V, Radha
V. EIF2AK3 mutations in South Indian children with permanent neonatal
diabetes mellitus associated with Wolcott-Rallison syndrome. Pediatr
Diabetes. 2014;15:313-8.
|
|
|
 |
|

