|
|
|
Indian Pediatr 2020;57: 68 -69 |
 |
Single Hepatitis B Booster Dose in High-risk Children with
Suboptimal Surface Antigen Antibody Responses After 3-dose
Primary Vaccine Series
|
|
Jia-Ming Low 1*,
Le-Ye Lee2,3,
Michelle Li-Nien Tan1,2,
Michelle Hong4
and Si-Min Chan1,2
1Khoo Teck Puat-National University
Childrens Medical Institute; 2Department of Paediatrics,
Yong Loo Lin School of Medicine; National University of Singapore;
3Department of Neonatology, National University Hospital,
Singapore and 4Signature Research Program - Emerging
Infectious Diseases, Duke-NUS Medical School; Singapore.
Email:
[email protected]
|
|
This was a descriptive study of 30
children born to HBsAG positive mothers between June 2009 and December
2013. All children had anti-HBs response
£100
IU/L after 3 doses of hepatitis B vaccine primary series. A single
booster dose led to hepatitis B surface antibody titers
³100
IU/L in (85%) of children.
Keywords: Immunization, Prevention,
Seroprotection.
|
|
Approximately 10% of infants are non-responders or
have suboptimal vaccine response with hepatitis B surface antibody
(anti-HBs) titers £100
IU/L three months post-3 dose hepatitis B vaccine series [1-4].
Controversy remains over the need for booster dose in suboptimal
responders with antibody levels 10-100 IU/L. None of the international
guidelines address this, especially in high-risk infants born to
hepatitis B chronic carrier mothers [1,5]. The study aimed to describe
the change in anti-HBs titers in infants born to hepatitis B carrier
mothers and anti-HBs titer of £100
IU/L after the 3 dose primary series; and to determine if for infants
with anti-HBs titer of 10-100 IU/L, a single booster of 10 µg hepatitis
B vaccine will increase the anti-HBs titers to >100 IU/L.
This was a descriptive study of children born between
June 2009 to December 2013, to hepatitis B surface antigen (HBsAg) -
positive mothers, at a tertiary university hospital in Singapore, with
anti-HBs response £100
IU/L after completing 3 doses of hepatitis B 10 µg vaccine given at
birth, and age of 1 month and 6 months. Vaccine response was defined
based on anti-HBs level done 3 months after completion of the third
vaccine dose viz. non-responder (anti-HBs <10 IU/L) or suboptimal
responder (anti-HBs ³10
IU/L but £100
IU/L). Occult HBV infection was defined as the presence of hepatitis B
infection with undetectable hepatitis B surface antigen (HBsAg) [6].
Demographic data and details of maternal HBV
infection were collected for all children. Baseline anti-HBs levels were
checked for children who were suboptimal responders before
administration of the fourth booster dose [intramuscular 10 µg
monovalent hepatitis B (Engerix B, GSK, Wavre, Belgium)]. Eight weeks
post-booster, HBsAg, HBV DNA, hepatitis B core antibody (anti-HBc) and
anti-HBs titres were measured. Children who were non-responders received
a repeat three dose vaccine series and were excluded from follow-up.
Children whose mothers had hepatitis C virus or HIV infection, or
children born before 37 weeks gestation, had a birthweight less than 2.5
kg, or known primary immuno-deficiency, were excluded. Informed consent
was obtained from their parents and assent from those older than 6
years. Study was approved by the National Healthcare Group Domain
Specific Review Board.
Data were analyzed with SPSS version 25.0.
Comparisons were done using Mann Whitney test, and significance was
taken as P<0.05.
Thirty-nine children (3 non-responders and 36
suboptimal responders) were eligible for the study; 30 (13 females) were
recruited (3 non-responders and 27 suboptimal responders). Mean (SD) age
at time of recruitment was 63 (31.5) months. Majority were Chinese
(80%). Mean (SD) birth weight was 3.22 (0.26) kg. Twenty-four were
breastfed until 9 months, 6 were born via Caesarean section.
Five (16.7%) mothers were HBeAg positive with HBV DNA
viral load of >200,000 IU/mL in their third trimester prior to starting
tenofovir. Two (6.7%) received tenofovir during the last trimester.
There was incomplete data for 9 children; 4 (13.3%) declined booster
vaccination and 5 (16.7%) declined blood tests post-booster for personal
reasons. Hence, 21 children had both pre and post-booster serological
results for analysis. No children had detectable HBV DNA or reactive
anti-HBc.
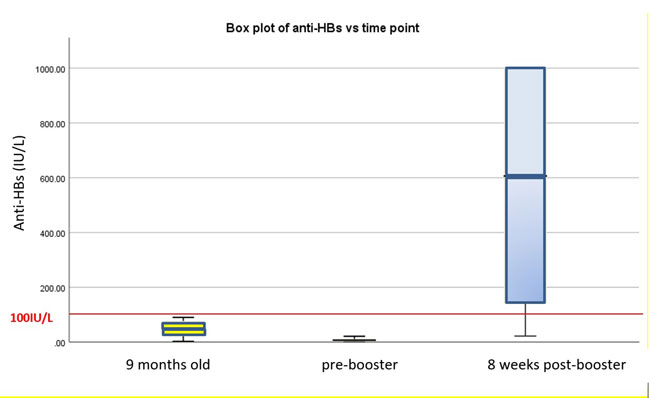
Median (IQR) anti-HBs titers 3 months after
completion of the primary vaccine series was suboptimal at 52 (22-77)
IU/L. Median (IQR) anti-HBs titers just prior to booster vaccine further
dropped to 7 (2-11) IU/L (P<0.05); 3 (10%) children had values
<10 IU/L. Mean (SD) time from completion of three-dose vaccine series to
booster vaccine was 62.6 (31.1) months. Median (IQR) anti-HBs titer rose
significantly to 606 (134-1000) (P<0.05)) IU/L post-booster
vaccine. Eighteen children (85.7%) demonstrated good anti-HBs response
(>100 IU/L) after the booster dose. Three children (14.3%) continued to
have suboptimal response post-booster vaccine (Fig. 1).
 |
|
Fig. 1 Box plot of anti-HBs versus time points.
|
Our study demonstrated that 85.7% of children with
suboptimal immune response post-primary series achieved anti-HBs >100
IU/L after a single booster dose, supporting the 2018 ACIP guidelines
that a single booster is sufficient instead of three repeat doses [5].
We also demonstrated that anti-HBs titers in infants
born to hepatitis B carrier mothers, and who had suboptimal antibody
titer three months after completing the three-dose primary series,
declined further over the next four years. Occult HBV infection was not
detected in this population as a cause of suboptimal response. Our small
series contributes to supporting evidence for a single booster, which is
cheaper and logistically easier, instead of repeating the three dose
series. A single booster may also increase adherence to vaccinations and
conserve public health resources involved in vaccine administration.
Acknowledgements: Dr Dimple Rajgor for her
assistance in literature search, and the writing, editing, formatting,
reviewing, and in submitting the manuscript for publication. Ms Ma Ting
for her assistance in data analysis.
Contributors: LJM: contributed in
investigation, data collection, curation and formal analysis, drafting
of the manuscript; LLY: conceptualized, designed, supervised the study,
made critical revisions to the drafted manuscript; TMLN: contributed in
investigation, data curation, and made critical revisions to the drafted
manuscript; HM: contributed in designing the study, formal data analysis
and made critical revisions to the drafted manuscript; CSM:
conceptualized, designed, supervised, investigated the study, made
critical revisions to the drafted manuscript. All authors approved the
final version of manuscript, and are accountable for all aspects related
to the study.
Funding: KTP-NUCMI Annual Grant call.
Competing interest: None stated.
References
1. WHO Publication. Hepatitis B vaccines: WHO
position paperrecommendations. Vaccine. 2010;28:589-90.
2. Li J, Hu J, Liang X, Wang F, Li Y, Yuan ZA.
Predictors of poor response after primary immunization of hepatitis B
vaccines for infants and antibody seroprotection of booster in a
Metropolis of China. Asia Pac J Public Health. 2015;27:NP1457-66.
3. Lee LY, Aw M, Rauff M, Loh KS, Lim SG, Lee GH.
Hepatitis B immunoprophylaxis failure and the presence of hepatitis B
surface gene mutants in the affected children. J Med Virol.
2015;87:1344-50.
4. Lee LY, Chan SM, Ong C, M Aw M, Wong F, Saw S,
et al. Comparing monovalent and combination hepatitis B vaccine
outcomes in children delivered by mothers with chronic hepatitis B. J
Paediatr Child Health. 2019;55:327-32.
5. Schillie S, Vellozzi C, Reingold A, Harris A,
Haber P, Ward JW, et al. Prevention of hepatitis B virus
infection in the United States: Recommendations of the Advisory
Committee on Immunization Practices. MMWR Recomm Rep. 2018;67:1-31.
6. Makvandi M. Update on occult hepatitis B virus infection. World J
Gastroenterol. 2016;22:8720-34.
|
|
|
 |
|

