|
|
|
Indian Pediatr 2020;57: 39-42 |
 |
Diagnostic Yield of Pneumococcal Antigen
Detection in Cerebrospinal Fluid for Diagnosis of Pneumococcal
Meningitis Among Children in China
|
|
Yong-Ping Xie 1,
Chun-Zhen Hua1*,
Hong-Jiao Wang1,
An-Na Sun2 and
Jue Shen3
From 1Division of Infectious Diseases,
2Clinical Laboratory Center, and 3Department of
Neurology, Children’s Hospital, Zhejiang University School of Medicine,
National Clinical Research Center for Child Health, Hangzhou, Zhejiang,
PR China.
Correspondence to: Dr Chun-Zhen Hua, Division of
Infectious Disease, Children’s Hospital, Zhejiang University School of
Medicine, Hangzhou 310003, P.R China.
Email: [email protected]
Received: October 14, 2018;
Initial review: March 25, 2019;
Accepted: October 23, 2019.
|
|
Objective: To determine the diagnostic accuracy of pneumococcal
antigen detection in diagnosis of pneumococcal meningitis in children.
Methods: Purulent meningitis was diagnosed according to European
Society for Clinical Microbiology and Infectious Diseases (ESCMID)
guideline between July 2014 and June 2016. Along with a cerebrospinal
fluid (CSF) culture, pneumococcal antigen detection in cerebrospinal
fluid (CSF) was performed, and further identification of pathogens was
done with 16S rDNA-PCR and high-throughput sequencing. Results:
CSF samples collected from 184 children (median age of 1.92 mo). CSF
culture was used as the gold standard. 46 (25%) had positive results for
culture and 10 (5.4%) were pneumococci; 34 (18.5%) were pneumococcal
antigen positive. The sensitivity and specificity of pneumococcal
antigen detection were 100% (95% CI: 89.4%–100%) and 86.2% (95% CI:
96.4%–99.9%), respectively. 92.3% (12/13) were confirmed by nucleic acid
detection to be pneumococci. Conclusions: Pneumococcal antigen
detection in CSF has adequate sensitivity and specificity in diagnosing
pneumococcal meningitis.
Keywords: Etiology, Rapid diagnosis,
Sensitivity, Specificity.
|
|
P
neumococcal meningitis is a life-threatening
disease with high incidence and case fatality rate (CFR) [1-2]. During
2000-15, the global incidence rate of PnM was 13/100,000 and CFR was
44%, and the burden was more in developing countries [1]. Early use of
sensitive antibiotics is extremely important for improving its prognosis
[3], which depends greatly on rapid etiological diagnosis. Usually,
clinicians depend mainly on the cerebrospinal fluid (CSF) culture, which
is time-consuming and can only detect the live bacteria in the specimen.
The detection of the pneumococcal antigen or nucleic acid can improve
the diagnosis of PnM [4-7]. Testing for pneumococcal urinary antigen
helped identifying pneumococci as pathogen in patients with invasive
pneumococcal diseases [4,6]; Immunochromatographic antigen test for the
detection of pneumococci had high sensitivity and specificity in CSF
samples from children with suspected bacterial meningitis [5]. However,
till recently, testing for pneumococcal antigen in CSF was not available
in China. The objective of this study was to determine the diagnostic
accuracy of pneumococcal antigen detection in diagnosis of pneumococcal
meningitis in children.
Methods
We enrolled patients with purulent meningitis and
hospitalized at our hospital between July 31, 2014 and June 30, 2016
after approval from institutional ethics committee. The inclusion
criteria included: (i) The clinical characteristics including
irritability, poor feeding, respiratory distress, marbling of skin and
hyper- or hypotonia in neonates or very young babies [8]; fever,
seizures, fontanell bulge, neck stiffness and vomiting in infants; and
headache accompanied by fever in old children [8]; and (ii)
mainly polymorphic leukocytes in CSF, elevated protein level, low
glucose concentration, low CSF to blood glucose ratio [8]. Patients who
had blood-tinged CSF that may affect the test results were excluded.
From each patients, 4-5 mL CSF specimens were collected and divided in
three portions: 1.0-1.5 mL for culture, 1-1.5 mL for cytology, and 1.5-2
mL for biochemistry, pneumococcal antigen detection and PCR.
Microorganism identification and antimicrobial susceptibility test were
performed by using the Vitek system (Mérieux, France). Pneumococcal
antigen was detected by using the BinaxNOW Streptococcus pneumonia
antigen detection kit (Alere, ME, USA).
Bacterial DNA was extracted from CSF, and bacterial
16S rDNA V3-V4 region was amplified by PCR using primer pairs: 341F: CCT
AYG GGR BGC ASC AG and 806R: GGA CTA CNN GGG TAT CTA AT. The PCR
products with sufficient quantity were collected and purified. An OTU
clustering analysis was carried out after high-throughput sequencing.
The results on culture and pneumococcal antigen detection in CSF in
patients with or without previous antibiotics were compared.
Statistical analysis: The collected data were
compared using the chi-square test. P<0.05 was considered to be
of statistical significance. Diagnostic accuracy testing was described
by calculating sensitivity and specificity.
Results
In this study, CSF samples were collected from 184
patients (36.4% neonates), aged from 1 day to 13 years and 8 months
(median age of 1.92 months). Only 46 (25%) had positive culture results;
with isolated bacteria being Escherichia coli (15 isolates),
pneumococci (10 isolates), Streptococcus agalactiae (7 isolates),
by Staphylococcus aureus (3 isolates), Enterococcus faecium
(3 isolates, Candida famata was isolated in one of them),
Streptococcus mitis (2 isolates), Listeria monocytogenes (2
isolates), Streptococcus sanguis (1 isolate), Enterobacter
cloacae (1 isolate), Haemophilus influenzae (1 isolate),
Acinetobacter baumannii (1 isolate), and C. famata (1
isolate). Fewer positive culture results were found in patients who had
received previous antibiotics when compared with those who had not (P<0.001);
but this difference was not seen for pneumococcal culture (P
=0.08).
Pneumococcal antigen was tested positive in 34
specimens (18.5%), which included these 10 positive pneumococci
cultures. No difference in the positivity rate of antigen detection was
found between those with history of previous antibiotics and those
without previous antibiotics (P=0.09) (Table I). 46
(25%) had positive results for culture and 10 (5.4%) were pneumococci;
34 (18.5%) were pneumococcal antigen positive. The sensitivity and
specificity of pneumococcal antigen detection were 100% (95% CI:
89.4%-100%) and 86.2% (95% CI: 96.4%-99.9%), respectively (Table
II).
TABLE I Pneumococcal Antigen Detection in Cerebrospinal Fluid of Patients With or Without Previous Antibiotics (N=184)
|
With previous
|
Without previous
|
|
antibiotics (n=136) |
antibiotics (n=48) |
|
All bacteria |
|
Culture positive
|
25 (18.4) |
21 (43.8) |
|
Culture negative
|
111 (81.6) |
27 (56.2) |
|
Pneumococcus |
|
Culture positive |
5 (3.7) |
5 (10.4) |
|
Culture negative |
131 (96.3) |
43 (89.6) |
|
Antigen positive |
29 (21.3) |
5 (10.4) |
|
Antigen negative |
107 (78.7) |
43 (89.6) |
TABLE II Pneumococcal Antigen Detection in Cerebrospinal Fluid in Patients With Suspected Pneumococcal Meningitis
|
Positive (n=10) |
Negative (n=174) |
|
Antigen positive, n (%) |
10 (100) |
24 (13.8) |
|
Antigen negative, n (%) |
0 |
150 (86.2) |
Twenty-one CSF specimens were selected for 16s
rDNA-PCR product sequence analysis. Among these were 13 positive and 8
negative for pneumococcal antigen testing. The distribution of the
bacteria at the species level based on OTU is shown in Fig. 1.
In one case with positive pneumococcal antigen, it was also positive for
E. faecium and C. famata in the CSF culture and the
abundance of pneumococcal OTU was low. As the sample with many species
of OTU may be contaminated, pneumococcal infection could not be
confirmed in this case.
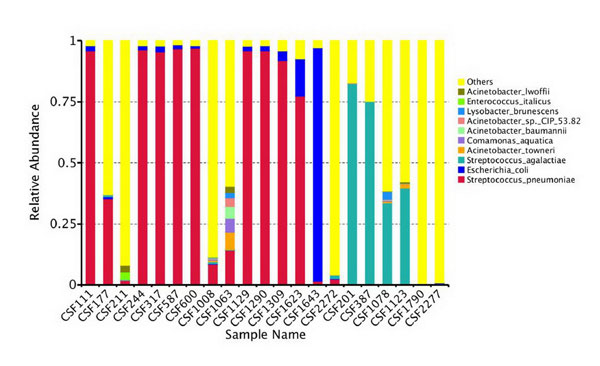 |
|
Fig. 1 The distribution of bacterial
species in 21 CSF samples by 16S rDNA-PCR high-throughput
sequencing and OTU clustering.
|
Finally, thirty-three patients were diagnosed with
PnM based on the combination result of pneumococcal antigen detection
and PCR [median (range) age: (2 mo 16 d-9 y 11 mo) 11.1 mo]. Thirty
(90.9%) were under the age of 5 years old, and one was a newborn (3%) 19
were boys. Twenty-eight were treated with
b-lactams or
b-lactams and other
antibiotics in combination for 1-27 days (median: 3 days) before they
received lumbar puncture. All of the 10 pneumococcal isolates were
resistant to penicillin and erythromycin but were sensitive to
ceftriaxone and vancomycin or linezolid. After admission to hospital,
all 33 patients were treated with b-lactams
antibiotics for 8-43 days (median: 18 days), including 81.8% (27/33) who
received another antibiotic in combination (24 with vancomycin and 3
with linezolid). Thirty-one patients (90.9%) were cured and the
incidence of complications was 27.3% (9/33). Two children did not
survive (2/33, 6.1%).
Discussion
The pneumococcal antigen test is a rapid diagnosis
method in the diagnosis of pneumococcal meningitis [7,10-11]; Its
advantage is the simplicity, rapidity, and usefulness in cases that have
already received prior antibiotics. The sensitivity and specificity in
our study were high, which was in accordance with the results from
previous studies that have evaluated the diagnostic accuracy by
detecting pneumococcal antigen in CSF specimens [7,9,10]. In the present
study, 72.7% of all (24/33) patients with pneumococcal meningitis were
missed when the diagnosis was based on the CSF culture, which was mainly
attributed to the fact that a majority of these patients had received
antibiotics before the sampling. The introduction of the pneumococcal
antigen test signi-ficantly improved the diagnosis of pneumococcal
meningitis in our study. One advantage of pneumococcal antigen test is
that the pneumococcal antigen might degrade slowly; it usually persists
in vivo until 7 days (90%) to 4-6 weeks (40-48%) after recovery
[11,12].
The pneumococcal antigen test was confirmed by PCR as
a method with high accuracy. Bacterial DNAs are still detectable by PCR
within several months after being killed by antibiotics; therefore, the
diagnosis of pathogen based on the pneumococcal antigen and nucleic acid
detection should be suggested in conjunction with clinical
manifestations. However, both positive results of pneumococcal antigen
detection and nucleic acid detection only provide the evidence of
pneumococcal infection, rather than ongoing infection. There was one
case positive for pneumococcal antigen testing but also positive for
E. faecium and C. famata in the CSF culture. As contamination
by E. faecium may lead to a false positive result of the
pneumococcal antigen test [6] and the abundance of penumococcal DNA was
not high, the exact pathogen in this case could not be determined and
was not considered as PnM.
There are certain limitations in this study. The CSF
specimen used for antigen detection and PCR were the same for
biochemical tests and thus had a certain risk of contamination. The 16S
rDNA sequencing could not be performed in all samples because of the
inadequate CSF volume, which may cause biased results. Further studies
are needed to confirm our conclusion with more patients.
Acknowledgment: Dr Ying-Jie Lu, Boston
Children’s Hospital, for critical reading of the manuscript.
Contributors: YPX: collecting of clinical
data; PCR of 16S rDNA; test the pneumococcal antigen; analysis and
interpretation of data; drafting the article; CZH: design of the
study; diagnosis of meningitis; analysis on 16S rDNA OTU; revising the
article critically for important intellectual content; HJW: diagnosis of
meningitis; collecting of cerebrospinal fluid (CSF) and clinical data;
ANS: isolation and identification of the bacteria;
drug-sensitivity test. JS: acquisition of consent and collecting
specimen from the patients, collection and analysis of the clinical
data, revising the article.
Funding: None; Competing interest:
None stated.
|
What This Study Adds?
•
Pneumococcal antigen detection
in the cerebrospinal fluid has adequate sensitivity and
specificity in diagnosing pneumococcal meningitis.
|
References
1. Eton V, Schroeter A, Kelly L, Kirlew M, Tsang RSW,
Ulanova M. Epidemiology of invasive pneumococcal and Haemophilus
influenzae diseases in Northwestern Ontario, Canada, 2010-2015. Int J
Infect Dis. 2017;65: 27-33.
2. Wahl B, O’Brien KL, Greenbaum A, Majumder A, Liu
L, Chu Y, et al. Burden of Streptococcus pneumoniae and
Haemophilus influenzae type b disease in children in the era
of conjugate vaccines: global, regional, and national estimates for
2000-15. Lancet Glob Health. 2018;6: e744-57.
3. Bewersdorf JP, Grandgirard D, Koedel U, Leib SL.
Novel and preclinical treatment strategies in pneumococcal meningitis.
Curr Opin Infect Dis. 2018; 31:85-92.
4. Hensby-Bennett S, Garland J, Philcox W, McCarthy
S, Playle V, Kesha K, et al. Rapid Streptococcus pneumonia
antigen detection on postmortem urine in a death due to pneumococcal
meningitis. Am J Forensic Med Pathol. 2019;40:269-72.
5. Alqayoudhi A, Nielsen M, O’Sullivan N, Corcoran M,
Gavin PJ, Butler KM, et al. Clinical utility of polymerase chain
reaction testing for Streptococcus pneumoniae in pediatric cerebrospinal
fluid samples: A diagnostic accuracy study of more than 2000 samples
from 2004 to 2015. Pediatr Infect Dis J. 2017;36:833-6.
6. Athlin S, Altun O, Eriksen HB, Ozenci V, Stralin
K. The Uni-Gold™ Streptococcus pneumoniae urinary antigen test: An
interassay comparison with the BinaxNOW Streptococcus pneumoniae test on
consecutive urine samples and evaluation on patients with bacteremia.
Eur J Clin Microbiol Infect Dis. 2015;34:1583-8.
7. Saha SK, Darmstadt GL, Yamanaka N, Billal DS,
Nasreen T, Islam M, et al. Rapid diagnosis of pneumococcal
meningitis: implications for treatment and measuring disease burden.
Pediatr Infect Dis J. 2005;24:1093 -8.
8. Van de Beek D, Cabellos C, Dzupova O, Esposito S,
Klein M, Kloek AT, et al. ESCMID guideline: diagnosis and
treatment of acute bacterial meningitis. Clin Microbiol Infect.
2016;22:S37-62.
9. Boulos A, Fairley D, McKenna J, Coyle P.
Evaluation of a rapid antigen test for detection of Streptococcus
pneumoniae in cerebrospinal fluid. J Clin Pathol. 2017; 70:448-50.
10. Picazo JJ, Contreras JR, Ríos E, Culebras E,
Rodríguez-Avial I, Méndez C, et al. Heracles Study Group. Rapid
diagnosis of invasive pneumococcal disease in pediatric population. J
Microbiol Methods. 2013;93:116-20.
11. Marcos MA, Jimenez de Anta MT, de la Bellacasa
JP, Gonzalez J, Martínez E, Garcia E, et al. Rapid urinary
antigen test for diagnosis of pneumococcal community-acquired pneumonia
in adults. Eur Respir J. 2003;21: 209-14.
12. Smith MD, Derrington P, Evans R, Creek M, Morris
R, Dance DA, et al. Rapid diagnosis of bacteremic pneumococcal
infections in adults by using the Binax NOW Streptococcus pneumoniae
urinary antigen test: A prospective, controlled clinical evaluation. J
Clin Microbiol. 2003;41:2810-3.
|
|
|
 |
|

