|
|
|
Indian Pediatr 2020;57: 34-38 |
 |
HIV-free Survival at the Age of 18 Months in
Children Born to Women With HIV Infection: A Retrospective
Cohort Study
|
|
Noopur Baijal, Anju Seth, Sarita
Singh, Garima Sharma, Praveen Kumar and Jagdish Chandra
From Department of Pediatrics,
Lady Hardinge Medical College, New Delhi, India.
Correspondence to: Dr Anju Seth,
Director Professor, Department of Pediatrics, Lady Hardinge Medical
College,
New Delhi, India.
Email: [email protected]
Received: March 25, 2019;
Initial review: April 15, 2019;
Accepted: October 04, 2019.
|
|
Objective: To assess HIV-free
survival and nutritional status of HIV-exposed infants. Methods:
This retrospective cohort study was conducted on infants born to woman
with HIV infection born at our Institute between January 2011 to March
2016, and followed using current National guidelines. HIV transmission
rate, HIV-free survival, and nutritional status were assessed 18 months
age. Results: Of the 155 infants, 10 (6.5%) died before 18 months
of age. Two of 145 surviving infants were confirmed HIV-positive, the
remaining were HIV-negative at 18 months (HIV-free survival 92.3%). Of
the 10 infants who died, one was confirmed HIV-positive and three
negative; the rest died before their HIV status could be ascertained.
HIV infection rate among the 149 infants for whom the test reports were
available was 2%. At 18 months age, 14% HIV-uninfected infants were
wasted, 28% stunted, and 3% had microcephaly. Conclusions:
Infants born to mothers with HIV managed as per the current National
guidelines have a good outcome at 18 months of age.
Key words: HIV exposure, infants,
Malnutrition, Outcome.
|
|
E
xposure to the same adverse environment places
HIV-exposed infants at a higher risk of morbidity and mortality
regardless of their own HIV status, as compared to infants born to women
without HIV infection [1,2]. The survival and health of these infants
are influenced by the feeding strategy adopted, higher exposure to
infections, and HIV-status of the infant himself [3,4]. A high mortality
in these children has previously been reported from this setting [5].
The current prevention of parent to child
transmission (PPTCT) guidelines by National AIDS Control Organization
(NACO), recommend lifelong anti-retroviral therapy (ART) to all pregnant
and breast-feeding women with HIV regardless of clinical or
immunological stage, anti-retroviral (ARV) prophylaxis to the baby, and
safe infant feeding practices. A well-defined protocol has also been
developed for care of the HIV-exposed infants [6]. The objective of this
work is to report outcome of HIV-exposed infants born at a tertiary-case
pediatric hospital, and provided standardized care as per the current
NACO protocol.
Methods
This retrospective cohort study was conducted in the
Pediatric Centre of Excellence in HIV care located at a public teaching
hospital in northern India. Infants born to women with HIV infection at
the linked hospital and registered in the PPTCT program at our Center
from January 2011 to March 2016 were included. We excluded infants born
at other hospitals and subsequently referred to our Centre, those
diagnosed with HIV after admission to our pediatric wards, or those who
never attended the Centre after birth at the linked hospital.
In accordance with the National guidelines, the HIV-
exposed infants are registered at birth in our Centre and given a
protocol-based care till 18 months of age [6]. This includes provision
for early HIV diagnosis, safe feeding counselling, and access to routine
infant care practices. Prior to January 2014, all women with HIV and
their newborns were given a single dose of nevirapine (SDNVP) during
labor and immediately after birth, respectively, in accordance with the
national guidelines at that time [7]. After January 2014, all pregnant
women with HIV are initiated on ART during pregnancy soon after
detection of their HIV status. Infants born to these women are started
on daily nevirapine prophylaxis at birth and continued for a minimum of
6 weeks [6]. This study included subjects registered both before and
after these changes in the National recommendations. Determination of
HIV status was done through HIV-1 DNA-PCR by dried blood spot (DBS) at
ages 6 weeks, 6 months, and six weeks after stopping breastfeeding.
Infants testing positive on DBS testing were re-tested for DNA-PCR on
whole blo od sample. In infants older than 18 months, serological tests
(3 rapid antibody tests) were done for HIV diagnosis.
For the current study, information on maternal and
infant characteristics was obtained from the records of all eligible
infants maintained at our Centre. The nutritional status of children was
determined by calculating Z-scores for weight for age (WFA), weight for
length (WFL), length for age (LFA) and head circumference for age (HFA)
using WHO growth reference standards [8].
The infants were considered to be HIV-infected if
they tested positive on DNA-PCR any time before 18 months, or were found
reactive on HIV serology at 18 months or beyond. They were considered
HIV-uninfected if they had a negative DNA-PCR test and were not
breastfeeding or had stopped it 6 weeks prior to the test, or had a
non-reactive HIV serological test at or after 18 months performed at
least 6 weeks after cessation of breastfeeding. The study was approved
by the Institutional Ethics Committee for Human Research.
Statistical analysis: The data were analyzed
using the SPSS statistical software package, Version 23. Chi square
test, unpaired t test and Mann-Whitney U test were used to compare
maternal and infant variables among HIV- uninfected infants at 18 months
and those who died.
Results
During the study period, 165 HIV-exposed infants were
born at the linked hospital. Among these, 155 mother-infant pairs who
were followed up at our centre till 18 months were eligible for the
study. The clinical characteristics of these mother infant dyads are
shown in Table I.
TABLE I Clinical Characteristics of Infants Born to Mothers with HIV Infection (N=155)
|
Characteristics |
No. (%) |
|
Male |
82 (53) |
|
Mother’s HIV diagnosis |
|
Before pregnancy |
72 (46) |
|
During pregnancy |
77 (50) |
|
After delivery |
6 (4) |
|
Mother’s therapy status at delivery |
|
*On triple ART |
123 (79) |
|
#Single dose nevirapine |
23 (15) |
|
No ART/ARV |
9 (6) |
|
Infant feeding status |
|
Exclusive breast feeding |
82 (53) |
|
Exclusive replacement feeding |
71 (46) |
|
Mixed feeding |
2 (1) |
|
Anthropometry at birth, mean (SD) |
|
WFL Z-score |
-1.2 (1.4) |
|
LFA Z-score
|
-0.7 (1.1) |
|
OFC Z-score |
-1.0 (1.1) |
|
Known HIV: infected before pregnancy: *68/72 and #4/72; HIV:
positive detected during pregnancy: *55/77 and #19/77; OFC:
Occipito-frontal circumference; LFA: Length for age; WFL: Weight
for length. |
Of the 155 infants, 10 (6.5%) died before 18 months
of age. Among the 145 surviving infants, two were confirmed
HIV-positive. The rest 143 (92.3%) were surviving and HIV-free at 18
months. Of the 10 infants who died before 18 months, one had positive
and three had negative HIV DNA-PCR at the age of 6 weeks (all 3 on
exclusive replacement feeds), while the rest died before their HIV
status could be ascertained (Fig. 1).
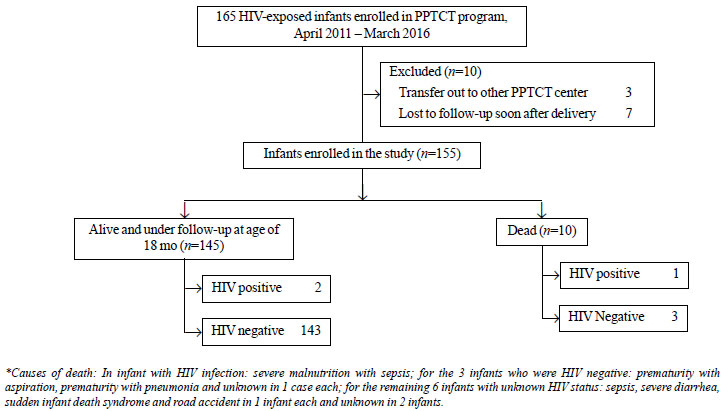 |
|
Fig. 1 Outcome of HIV-exposed infants enrolled in
the study.
|
HIV infection was reliably excluded in 146/155
infants (143 of those alive and 3 of those who died) while it was
diagnosed in 3 infants (2 of those alive and 1 among those who died).
Thus, HIV infection rate in the infants for whom the HIV test reports
were available was 2% (3/149). On analyzing the data before (n=93)
and after (n=62) the change in PPTCT guidelines, no significant
difference was found in terms of HIV-free survival (92.5% vs
91.9%; P=0.9); or HIV transmission rate (2.2 vs 1.7 %; P=0.82).
The outcome in terms of survival and status of HIV
infection stratified as per various maternal and infant factors is
presented in Table II. Details of the three infants who
were diagnosed with HIV infection are presented Web Table I.
TABLE II Outcome of Infants Stratified According to Maternal and Infant Characteristics (N=155)
|
Characteristics |
Total
|
Alive at 18 mo (n=145)
|
Death
|
|
|
Infected (n=2) |
Not infected (n=143) |
(n=10) |
|
Maternal ART/ARV at the time of delivery, n (%) |
|
On ART |
123 (79) |
2 (100) |
114 (80) |
7 (70) |
|
On ARV prophylaxis |
23 (15) |
0 |
23 (16) |
0 |
|
Not on ART/ARV or started after delivery |
9 (6) |
0 |
6 (4) |
3 (30) |
|
*ART duration, mo
|
6 (2.3-24) |
17.5 (1-17.5) |
6 (3-23.3) |
2 (0.8-35) |
|
#Vaginal delivery |
70 (45) |
2 (100) |
60 (42) |
8 (80) |
|
Maternal CD4 count, cells/mm3, mean (SD) |
359 (191.9) |
259 (55.2) |
365.7 (196.1) |
300 (142.9) |
|
‡Infant prophylaxis, n (%) |
|
Single dose Nevirapine |
40 (26) |
|
37 (26) |
3 (30) |
|
Nevirapine for 6 wk |
86 (55) |
1 (50) |
81 (57) |
4 (40) |
|
Nevirapine ≥12 wk |
28 (18) |
1 (50) |
24 (16.8) |
3 (30) |
|
Birthweight (kg), mean (SD) |
2.6 (0.5) |
2.6 (0.6) |
2.6 (0.4) |
2.1 (0.7) |
|
$Feeding during first 6 mo, n (%) |
|
Exclusive breastfeeding |
82 (53) |
2 (100) |
76 (53) |
4 (40) |
|
Replacement feeding |
71 (46) |
|
65 (45) |
6 (60) |
|
**Maternal CD4 < 350 cells/mm3, n (%)^ |
70 (56) |
2 (100) |
61 (53) |
7 (78) |
|
*Value in median (IQR); HIV-uninfected surviving
infants and dead infants; P values of #0.02 and **0.002;
^Available for 126 infants including the 2 infected infants, 115
alive uninfected infants and 9 dead infants; ‡One infant did not
receive prophylaxis; $two infant were on mixed feeding. |
At the age of 18 months, Z scores for WFL (n=108),
LFA (n=108) and OFC (n=106) were -0.6 (1.2) , -1.2 (1.2),
and -0.9 (1.2), respectively. At that time, 15 (14%) of uninfected
infants were wasted, 30 (28%) infants were stunted, and 3 (3%) had
microcephaly.
TABLE III Details of Infants Detected HIV-Infected
|
Case
|
Mother’s details
|
Infant
|
Feeding |
Infant HIV testing |
Outcome
|
|
number |
Prophylaxis |
Pre-delivery
|
prophylaxis |
(total duration) |
6 wk |
6 mo |
18 mo |
|
| |
(duration before |
CD4 counts
|
|
|
|
|
|
|
| |
delivery) |
(cells/mm3) |
|
|
|
|
|
|
|
1 |
ART (2 y 10 mo)
|
220 |
Nevirapine for |
Breastfeeding |
DBS*
|
DBS |
Serology |
Alive/ on ART
|
|
|
|
6 wk |
(13 mo) |
negative |
negative |
positive |
|
|
2 |
ART (1 mo) |
298 |
Nevirapine for |
Breastfeeding |
Not done
|
Serology
|
Serology |
Alive/ on ART |
|
|
|
12 wk |
(16 mo) |
|
negative |
positive |
|
|
3 |
ART (4 mo) |
334 |
Single dose
|
Replacement |
DBS |
- |
- |
Died at 3 mo of |
|
|
|
nevirapine |
feeding |
positive |
|
|
age (severe sepsis) |
|
*DBS: Dried blood sample. |
Discussion
The present study has documented HIV infection rate
and HIV-free survival among infants of women with HIV infection managed
as per national PPTCT strategy. Parent to child transmission rate in the
present study was 2%, with an overall ARV cover in HIV positive mothers
of 94% (ART 79%, SDNVP 15%). We have previously reported a rate of 14.8%
when the ARV cover in HIV-infected mothers was only 61.5% [5]. The
current transmission rate is also much less when compared to studies
from Africa [9,10], as well as few studies from India where the
transmission rate has varied between 8-19% [11,12]. These studies were
conducted during the time when most mothers received SDNVP, ART being
limited only to those eligible as per their clinical/immunological
criteria. In a recent study, where 37% of HIV-infected pregnant women
received ART, and 63% SDNVP, Seenivasan, et al. [13] have
reported a HIV transmission rate of 4%. Another study from this region,
where 92% of enrolled women were getting either ARV prophylaxis or ART,
a HIV transmission rate of 3.4% was reported [14]. The results of the
current and these other recent studies from India show that with current
robust PPTCT strategy, the HIV transmission rate in India is approaching
the rate observed in developed countries (1-2%) [15,16].
We observed a 92.3% HIV-free survival at 18 months of
age, similar to a recent study from Rwanda [17] that reported a 24-month
HIV-free survival of 93.2% in breastfeeding infants of HIV-positive
mothers on lifelong ART. A systematic review including 18 studies
published between 2005 to 2015 provided a pooled estimate of 18-month
HIV-free survival of 89.0% with 6 months ART and 96.1% with lifelong ART
[18]. The authors found that the HIV-free survival, though higher in the
breastfeeding group, did not significantly differ by feeding patterns.
Similar findings were also observed in the present study. At 18 months,
the prevalence of wasting and stunting among infants was no different
from that reported among Indian children of this age group as per NFHS-4
[19].
Several reasons contribute towards better outcome of
HIV-exposed infants in terms of survival, HIV transmission and
nutritional status in the current as compared to our previous study [5].
Unlike the previous study, the present work excluded infants diagnosed
as HIV-exposed/infected after birth. A much higher proportion of mothers
were on ART (79%) as compared to the previous study (17.4%). Provision
of a protocol-based care with focus on repeated counselling to optimize
health of mother-infant dyad also contributed to the improved outcome.
Due to a small number of infants who acquired HIV
infection or died, our results give limited information regarding
predictors of HIV infection transmission/mortality in HIV-exposed
infants. As routine viral load was not introduced in the national
protocol during this study period, maternal viral load, that directly
impacts upon the HIV transmission rate, could not be assessed.
We conclude that implementation of the current PPTCT
strategy, which includes lifelong ART to all HIV-infected pregnant and
breastfeeding women with ARV prophylaxis to their infants, and a
structured follow up of HIV-exposed infants, has remarkably improved the
outcome of these infants.
Contributors: NB: managed the cases, recorded the
information and drafted the paper; AS: conceptualized the paper, drafted
and edited the manuscript and was the consultant in patient management.
She will be the corresponding author for this work; SS: contributed
towards design of the work, data analysis and manuscript preparation;
GS: provided clinical care to study subjects, and contributed towards
record keeping and manuscript preparation; PK, JC: consultants in
patient management and helped in drafting /editing the paper. All
authors gave their final approval for the submitted manuscript.
Funding: None; Competing interest: None
stated.
|
What This Study Adds?
•
Implementation of the current
Prevention of parent-to-child transmission (PPTCT) strategy and
a structured follow up of HIV-exposed infants results in an
HIV-free survival matching that observed in more developed
countries.
|
References
1. Landes M, Lettow MV, Chan AK, Mayuni I, Schouten
EJ, Bedell RA. Mortality and health outcomes of HIV exposed and
unexposed children in a PMTCT cohort in Malawi. PLoS One. 2012;7:e47337.
2. Slogrove A, Reikie B, Naidoo S, Beer CD, Ho K,
Cotton M, et al. HIV exposed uninfected infants are at increased
risk for severe infections in the first year of life. J Trop Pediatr.
2012;58:505-8.
3. Ram M, Gupte N, Nayak U, Kinikar AA, Khandave M,
Shankar AV, et al. Growth patterns among HIV-exposed infants
receiving nevirapine prophylaxis in Pune, India. BMC Infect Dis.
2012;12:282.
4. Sobze MS, Wadoum RG, Temgoua E, Donfack JH, Ercoli
L, Buonomo E, et al. Evaluation of the nutritional status of
infants from mothers tested positive to HIV/AIDS in the health district
of Dschamg, Cameroon. Pan Afr Med J. 2014;18:91.
5. Seth A, Chandra J, Gupta R, Kumar P, Aggarwal V,
Dutta A. Outcome of HIV exposed infants: Experience of a regional
pediatric center for HIV in North India. Indian J Pediatr. 2012;79:188-93.
6. Updated guidelines for Prevention of Parent to
Child Transmission (PPTCT) of HIV using multi drug Anti-retroviral
regimen in India. December, 2013. National AID Control Organization.
Available from http://naco.gov.in/sites/default/files/National_Guidelines_for_PPTCT_
0.pdf. Accessed April 04, 2018.
7. Guidelines for HIV care and treatment in infants
and children 2006. Available from http://apps.who.int/medi cinedocs/documents/s18022en/s18022en.pdf.
Accessed April 04, 2018.
8. WHO child growth standards: length/height-for-
age, weight for-age, weight-for-length, weight-for-height and body mass
index-for age: methods and development. Geneva: World Health
Organization, 2006. Available from
http://www.who.int/childgrowth/standards/technical_ report.pdf.
Accessed April 04, 2018.
9. Birlie B, Diriba TA, Sisay K, Gurmessa A, Seyoum
D, Tadesse M. Mother to child HIV transmission and its predictors among
HIV-exposed infants: A retrospective follow-up study in southwest
Ethiopia. J AIDS Clin Res. 2016;7:605.
10. Ng’ambi WF, Ade S, Harries AD, Midiani D, Owiti
P, Takarinda KC, et al. Follow-up and programmatic outcomes of
HIV-exposed infants registered in a large HIV centre in Lilongwe,
Malawi: 2012–2014.Trop Med Int Health. 2016;21:995-1002.
11. Mukherjee S, Ghosh S, Goswami DN, Samanta A.
Performance evaluation of PPTCT (Prevention of parent to child
transmission of HIV) programme: An experience from West Bengal. Indian J
Med Res. 2012;136:1011-9.
12. Malpani P, Biswas M, Kale V. Outcome of children
born to human immunodeficiency virus positive mothers- A retrospective
study. Indian Journal of Child Health. 2016; 3:244-7.
13. Seenivasan S, Vaitheeswaran N, Seetha V,
Anbalagan S, Karunaianantham R, Swaminathan S. Outcome of prevention of
Parent-To-Child Transmission of HIV in an urban population in Southern
India. Indian Pediatr. 2015; 52:759-62.
14. Dwivedi S, Jahan U, Dwivedi GN, Gupta N, Verma K,
Sharma B, et al. Perinatal outcome in HIV infected pregnant women
at tertiary care hospital in North India: Eleven years retrospective
study. International Journal of Recent Scientific Research.
2017;8:16801-5.
15. Townsend CL, Cortina-Borja M, Peckham CS, de
Ruiter A, Lyall H, Tookey PA. Low rates of mother-to-child transmission
of HIV following effective pregnancy interventions in the United Kingdom
and Ireland, 2000-2006. AIDS. 2008;22:973-81.
16. Centers for disease control and prevention:
Achievements in public health. Reduction in perinatal transmission of
HIV infection-United States, 1985–2005. MMWR Morb Mortal Wkly Rep.
2006;55:592-7.
17. Gill MM, Hoffman HJ, Ndatimana D, Mugwaneza P,
Guay L, Ndayisaba GF, et al. 24-month HIV-free survival among
infants born to HIV-positive women enrolled in Option B+program in
Kigali, Rwanda: The Kabeho study. Medicine (Baltimore). 2017;96:e9445.
18. Chikhungu LC, Bispo S, Rollins N, Siegfried N,
Newell ML. HIV-free survival at 12 - 24 months in breastfed infants of
HIV-infected women on ART. Trop Med Int Health. 2016;21:820-8.
19. International Institute for Population Sciences
(IIPS) and ICF. 2017. National Family Health Survey (NFHS-4), 2015-16:
India. Mumbai: IIPS.
|
|
|
 |
|

