|
|
|
Indian Pediatr 2020;57:25-33 |
 |
Normative Data of
Infant Pulmonary Function Testing: A Prospective Birth Cohort
Study from India
|
|
Prawin Kumar, Aparna Mukherjee, Shivani Randev, Kana Ram Jat, Rakesh
Lodha and Sushil K Kabra
From Department of Pediatrics, All India Institute of Medical
Sciences, New Delhi, India.
Correspondence to: Dr SK Kabra, Professor, Pediatrics Pulmonology
Division, Department of Pediatrics, All India Institute of Medical
Sciences, Ansari Nagar, New Delhi 110 029, India.
Email: [email protected]
Received: January 18, 2019;
Initial review: July 10, 2019;
Accepted: October 11, 2019.
|
Objective: To develop a normal reference range of
Infant pulmonary function test (IPFT) indices for Indian children.
Design: Prospective birth cohort study.
Setting: Division of Pediatric Pulmonology of a
tertiary-care institute in India from August 2012 to March 2017.
Participants: All neonates born at the institute
during the study period were screened for eligibility.
Measurement: IPFT at baseline and every 6-month
until 36-months of age.
Main Outcome Measure(s): Tidal breathing
flow-volume loop (TBFVL), Rapid thoracoabdominal compression (RTC), and
Raised volume RTC (RVRTC) indices at baseline and follow-up.
Results: 310 newborns were enrolled in the
cohort; 281 of them (169 male) had completed 36-months of follow-up at
the end of the study period. There was no influence of gender on the
baseline IPFT indices. Tidal volume per unit body weight (VT/kg)
significantly increased from baseline to 36 months of age (P<0.001)
while the peak ratio (tPTEF/tE) initially decreased in
first 18-months of age (P<0.001), after that returned to the
baseline value by 36 months of age. RTC indices did not change
significantly from baseline values. In RVRTC, the ratio of forced
expiratory volume in 0.5s to forced vital capacity (FEV0.5/FVC) was
significantly decreased from baseline to 36 months of age (P=0.002).
Conclusions: Normal values for various IPFT
indices for TBFVL, RTC, and RVRTC from neonates to the age of 36-month
are provided. These data may be used as normative data for healthy
neonates and children of Indian origin.
Keywords: Indices, Rapid thoracoabdominal compression, Tidal
breathing flow-volume loop.
|
|
W
hile pulmonary function testing (PFT) is well
established for older children (>5 years) and adolescents, it is still
evolving for use in infants and preschool children [1]. Sophisticated
equipments are now commercially available, which can measure pulmonary
function even in premature babies [2].
PFT in infants (IPFT) may contribute to a better
understanding of the nature and severity of respiratory illness,
progression of the disease, and monitoring response to therapy [3].
Serial measurements of lung function since birth, especially in
high-risk infants, may be helpful in recognition of early deviation from
the normal pattern of lung development. Longitudinal studies have shown
that many of the chronic respiratory disorders have their origin in
childhood; hence, intervention at this stage may have an impact on the
management of the chronic respiratory disease [4,5].
The measurement of PFT in infants and preschool
children is a major challenge [6,7]. The values of pulmonary function
indices vary with age, sex, body size, and ethnic groups [8,9].
Currently, there is lack of multiethnic global reference range for IPFT
indices, so the development of regional ethnicity-specific normative
data is the need of the hour, which will definitively expand its use in
clinical practice [6].
In this birth cohort, we performed IPFT from birth to
36 months of age. The normative data for various indices were generated,
which may be used as reference range in a similar population.
Methods
This prospective birth cohort study was conducted in
the Department of Pediatrics of a tertiary-care institute in Delhi,
India from August 2012 to March 2017. The study was approved by the
Institutional Ethics Committee. Written informed consent was taken from
the parents/guardians. All neonates born at the institute during the
study period were screened for eligibility. The inclusion criteria were
age £4 weeks,
full-term (³37
weeks of gestation), and appropriate for gestational age (weight 10th-
90th centile) babies [10].
The exclusion criteria were any perinatal insults (e.g. birth
asphyxia, meconium aspiration, any amount of respiratory distress
requiring respiratory support, pathological hyperbilirubinemia or
seizure), known major congenital birth defect, required parenteral
antibiotic or fluid, neonatal cholestasis, chronic kidney disease or
inborn error of metabolism, and mother having antepartum or postpartum
hemorrhage, preeclampsia or eclampsia, HIV infection, or parents’
refusal for regular follow-up for three years. The enrolled babies were
followed-up every 6 months (±8 weeks) and also whenever they had an
acute respiratory infection or any other acute condition. The babies
were clinically examined, and anthropometric measurements (weight,
length, head circumference) were recorded at enrolment and each visit.
The IPFT were performed with Exhalyzer D (Eco Medics
AG, Duernten, Switzerland) as per the American Thoracic Society/European
Respiratory Society Task Force recommendations [11-16]. IPFT included
tidal breathing flow-volume loop (TBFVL), rapid thoracoab-dominal
compression (RTC), and raised volume rapid thoracoabdominal compression
(RVRTC) maneuvers (Web Fig. 1). All the IPFT maneuvers
were performed in the Pediatric Pulmonary Function Laboratory, which is
well equipped with a central supply of oxygen and resuscitation
equipment. The equipment was calibrated daily for atmospheric
temperature and pressure and volume with a 100 mL calibration syringe
(M30.9011) provided by the manufacturer. Weight and length/height were
recorded using standard methods [17]. IPFT was postponed for 2-4 weeks
if the child had an acute respiratory infection.
At enrolment and 6 months of age, IPFT were performed
either in awake or natural sleep state after breastfeeding. Beyond six
months of age, syrup Triclofos (25-50 mg/kg/dose) was used for sedation,
whenever required. The maneuvers were performed in the supine position.
Baseline TBFVL was completed within four weeks of birth, while RTC and
RVRTC were first performed once the child was
³8 kg as per
recommendations of the manufacturer of IPFT equipment. IPFT were
repeated at every follow-up visit. The IPFT indices and their
physiological interpretation are described in
Web Table I.
Statistical analysis: Clinical information during
each visit was recorded manually into case record form; data were then
entered into Microsoft Access software. IPFT data were automatically
stored in software (SPIROWARE, Eco Medics AG, Duernten, Switzerland)
after each procedure; data of each individual were extracted and managed
in Microsoft Access software. Data were analyzed using Stata software
v.13 (Stata Corp, College Station, TX, USA). Quantitative variables were
summarized using mean and standard deviation if normally distributed;
for skewed distribution, median (interquartile range) was used.
Chi-square test was used for the analysis of categorical data. The
change in IPFT indices from birth to 36 months of age were calculated
with the Wilcoxon sign-rank test. Comparison of IPFT indices between
gender and other parameters was analyzed with Wilcoxon rank-sum test
(Mann-Whitney Test). A P-value of <0.05 was considered
significant. For multivariate analysis, mixed-effects linear regression
analysis was performed; IPFT indices were taken as the dependent
variable, individual subjects as the random effect and gender, weight,
length, and age as fixed effect. The LMS chartmaker Pro (Medical
Research Council, UK) was used to model the expected median (µ or M),
the coefficient of variation ( s
or S), and skewness (l
or L) and to smooth the centile curves for IPFT
indices against age, utilizing the method described by Cole TJ, et al.
[18]. The goodness of fit for the model used was tested by the Q curve
[18].
Results
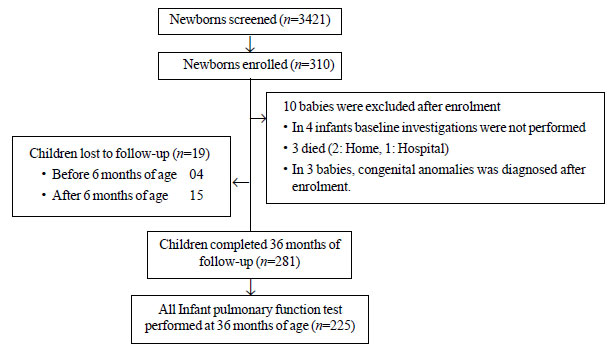
A total of 3412 neonates born in our institute from
August 2012 to May 2014 were screened for eligibility; 310 neonates
fulfilled the inclusion criteria and were enrolled. The median (IQR) age
at enrolment was 4 th (3rd,
5th) postnatal day. A total
of 281 children (90.6%) had completed 36 months of age by 31
March 2017; all IPFT (TBFVL, RTC and RVRTC) at
this age were successfully performed in 225 infants (54.5% males) (Fig.
1). The mean (SD) birthweight and length were 2.6 (0.6) kg and 47.7
(6.6) cm, respectively. Other demographic characteristics of enrolled
children are summarized in Table I. TBFVL, RTC, and RVRTC
were successfully performed in 1705, 948, and 875 occasions (baseline
and follow up visits), respectively. The 5th,
25th 50th,
75th, and 95th
centile values of TBFVL and RTC as per the age, sex, mean weight and
length/height of the enrolled children are described in Table
II and III. The same for RVRTC indices are described in
Table IV.
TABLE I Demographic Characteristics of Enrolled Infants (N=310)
|
Characteristics |
Values* |
|
Females |
141 (45.5) |
|
*Birthweight, g |
2648 (689) |
|
*Length, cm |
47.7 (6.6) |
|
*Gestation, d |
267.9 (22.6) |
|
Mode of delivery |
|
|
Vaginal |
185 (59.6) |
|
Caesarean |
101 (32.5) |
|
Instrumental |
24 (7.8) |
|
#Age at enrolment, d |
4 (3, 5) |
|
Age of parents, y |
|
|
Mother |
26.5 (3.9) |
|
Father |
30.5 (4.3) |
|
Family members in house |
5.3 (2.8) |
|
Urban accommodation |
277 (89.3) |
|
Smoking at home |
93 (30) |
|
Pets at home |
31 (10) |
|
Family history of allergy |
144 (46.4) |
|
Asthma |
82 (26.5) |
|
Allergic rhinitis |
93 (33.1) |
|
Atopic dermatitis |
32 (10 .3) |
|
All values in no. (%) except *mean (SD) or #median
(IQR). |
|
TABLE II Normal Values (Percentiles) of
Tidal Breathing Flow Volume Loop Indices
|
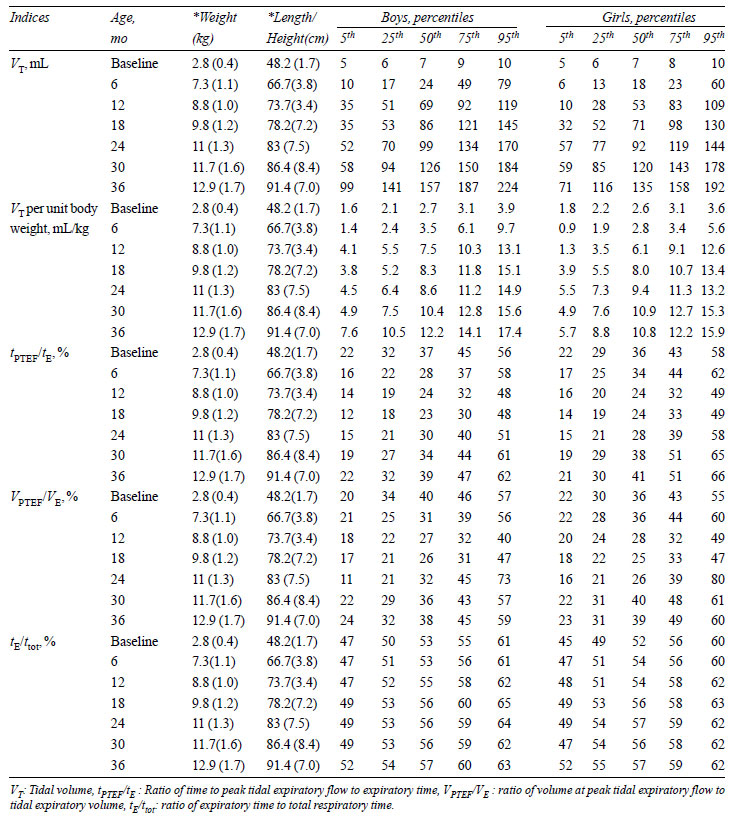 |
|
TABLE III Normal Values (Percentiles) of
Rapid Thoracoabdominal Compression Indices
|
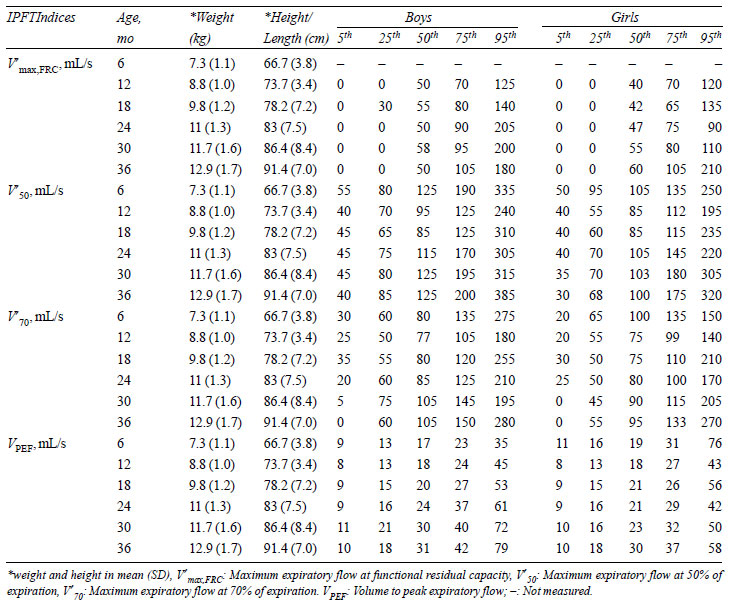 |
|
TABLE IV Normal Values of Raised Volume
Rapid Thoracoabdominal Compression Indices
|
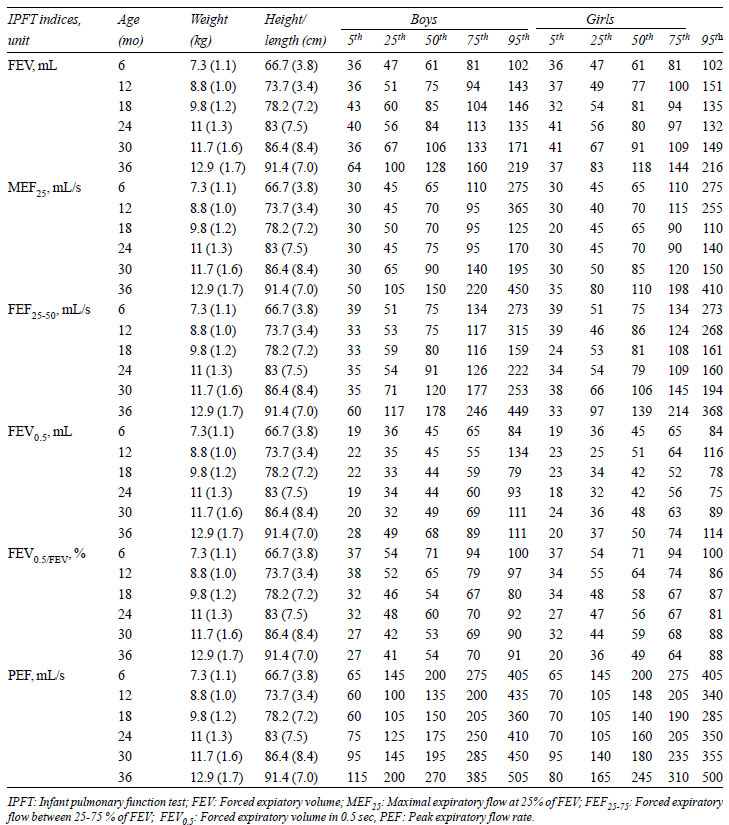 |
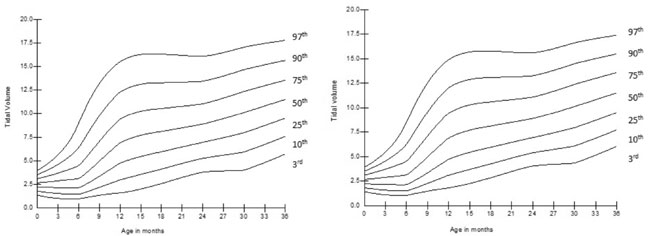
Tidal volume (VT)
per unit body weight (VT/kg)
increased significantly from birth to 36 months of age (P<0.001),
which was more prominent in the first 12 months of age (P<0.001).
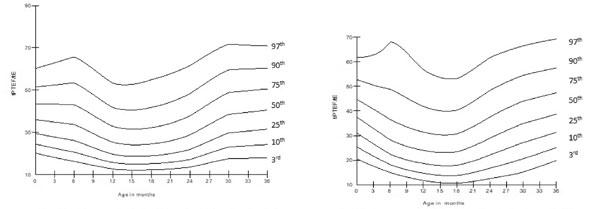
The ratio of time to attained peak expiratory flow to expiratory time (tPTEF/tE)
decreased significantly (P<0.001) from baseline till 18 months of
age, then it began to increase (P<0.001) to achieve the baseline
value at 36 months of age. VPTEF/VE
also had decreasing trends from baseline to 18
months (P<0.001) then it gradually increased to achieve the
baseline value by 36 months of age. The smoothed centile curves for VT
and tPTEF/tE
are given in Fig. 2 and 3. At
baseline, there was no significant association of gender with IPFT
indices. However, on follow up, VT
was significantly more in boys at age of 6 months (P<0.001),
12 months (P<0.001), 18 months (P=0.02) and 36 months (P<0.001).
The tPTEF/tE
was significantly more in girls at 6 months (P=0.004)
and 30 months (P=0.04) of age; for other ages it was similar in
both sex. VPTEF/VE
was significantly greater in girls at 6 months (P=0.004)
and 30 months (P=0.02) of age. The tE/ttot
was similar in both sexes at all ages.
 |
|
Fig. 1 Enrolment and follow-up of the
study cohort.
|
 |
|
Fig.2 (a) Change in tidal volume (VT)
with age in girls; (b) Change in tidal volume (VT) with age in
boys.
|
 |
|
Fig. 3 (a) Change in ratio of time to
attained peak tidal expiratory flow to expiratory time (tPTEF/tE )
with age in girls; (b) Change in ratio of time to attained peak
tidal expiratory flow to expiratory time (tPTEF/tE )
with age in boys.
|
 |
|
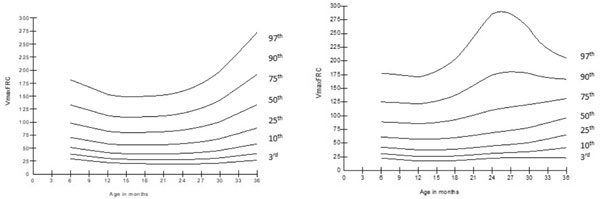
Fig. 4 (a) Change in maximum
expiratory flow at functional residual capacity (V¢max,FRC
) with age in girls; (b) Change in maximum expiratory flow at
functional residual capacity (V¢max,FRC
) with age in boys.
|
In RTC, V¢maxFRC,
V¢50,
and V¢70
did not significantly differ from baseline to 36
months of age, while VPEF
gradually increased from baseline to 36 months of age (P<0.001).
The smoothed centile curves for V¢maxFRC
are presented in Fig. 4. V¢max,FRC,
V¢50,
V¢70,
and VPEF
were similar in both sexes at all ages except at 30
months, where V¢70
(P=0.01) and VPEF
(P<0.001) were significantly higher in
boys. In RVRTC, there were minor increases in FVC, FEV0.5,
FEF25-75, and MEF25
from baseline till 24 months of age; after that,
there were significant increases till 36 months of age (P<0.01).
 |
|
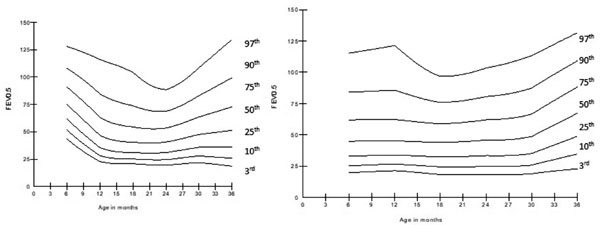
Fig. 5 (a) Change in forced
expiratory volume in 0.5 sec (FEV0.5 )
with age in girls; (b) Change in forced expiratory volume in 0.5
sec (FEV0.5 ) with age in boys.
|
PEF initially decreased from baseline to 12 months of
age (P<0.001) then remained constant till 24-months of age,
increasing again till 36 months of age (P<0.001). FEV0.5/FVC
was significantly decreased from baseline to 36-months of age (P=0.002),
more during baseline to 18 months of age (P<0.001). The smoothed
centile curves for FEV0.5
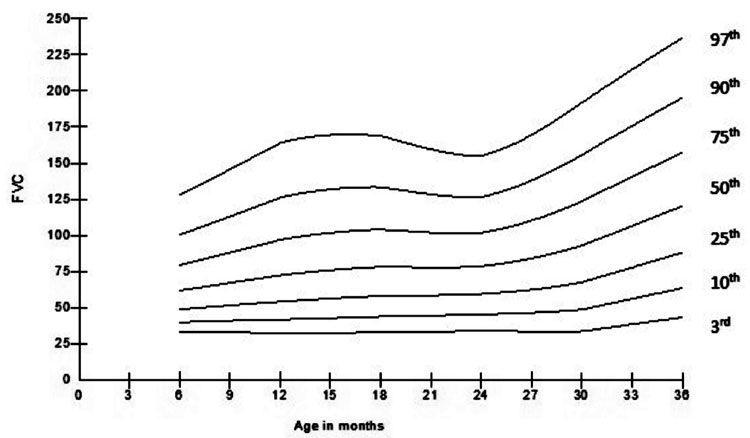
and FVC are represented in Fig. 5 and 6.
 |
|
Fig.6 Change in forced vital capacity
(FVC) with age.
|
RVRTC indices were similar in both sexes at all ages
except at 36-months, where FEV, MEF25,
FEF25-75, FEV0.5
and PEF, were significantly higher in boys (P=0.04,
0.01, 0.01, <0.001 and 0.02, respectively). FEV0.5/FVC
remained similar in both sexes at all ages.
On multivariate analysis, length/height, body weight,
gender, and age were significantly associated with V T.
tPTEF/tE
was significantly associated with gender, with
values at any point being higher for females in comparison to males,
after adjusting length/height and bodyweight.
Discussion
IPFT is now widely used in the research context;
however, its use in a clinical setting is still restricted by the
non-availability of regional and ethnicity-specific reference values. In
this prospective birth cohort study, we obtained normative data for
TBFVL for neonates to 36 months of age, and RTC and RVRTC from 6 months
to 36 months of age.
The major limitation of this study is that subjects
were recruited from a single center, which may not be truly
representative of the entire population. However, being a tertiary-care
institute, subjects were referred from all around the country. Another
limitation is that neonates with a family history of smoking or allergy
were not excluded in this study, as we considered that they would be
normally distributed, though to a different extent, in any given
population.
The major strength of this study is the meticulously
planned prospective birth cohort design of the study with frequent and
regular follow-up. IPFT were performed as per ATS/ERS recommendation.
Until one year of age, in the majority of the infants, TBFVL were
performed without sedation. As there was a significant difference
between genders at a particular age, so gender-specific data have also
been presented. Centile curves have been constructed using the LMS
method.
In comparison to studies on Caucasian population,
V T/kg was markedly lower
at baseline in this cohort; however, it gradually increased with age,
and by 12 months it became comparable to global values [19,20]. The rest
of the TBFVL indices at baseline were comparable with other studies
[19-21]. In this cohort, tPTEF/tE
and VPTEF/VE
were highest at baseline and then gradually
decreased until 18 months of age. A study from Switzerland in 342
infants had also observed that tPTEF/tE
gradually decreased in the first year of life
[20]. However, a prospective birth cohort study from Taiwan did not
observe any significant change in VT,
tPTEF/tE
and VPTEF/VE
from 5 to 26 months of follow-up [7]. Furthermore,
in the present study, there was no significant influence of gender at
baseline IPFT indices; however, on follow up, many of these indices
varied significantly with gender. A study from Norway did not find any
significant influence of gender on the baseline tidal expiratory volume;
however, they observed that tidal flow and flow ratio were significantly
higher in males in comparison to female babies [21]. Another study from
Taiwan also did not observe any sex-related difference in IPFT indices
[7]. V¢max,FRC
in this cohort remained similar throughout the
follow-up period with no influence of gender. Studies from Taiwan [7],
US [22], and a multicentric study from London, Indianapolis, and Boston
[23] had observed that V¢max,FRC
correlates significantly with the height of the
children. The measurement of V¢max,FRC
depends on accurate determination of FRC as a
volume landmark, which is highly variable, especially in younger
children, and this is a significant limitation in RTC measurement [24].
In this study, jacket pressure was kept fixed at 10 kPa while in other
studies, it was used in the incremental range from 2-10 kPa [22,25].
Hence, it might be responsible for the deviation of our finding for V¢max,FRC
with age from other studies.
In this cohort, most of the RVRTC indices viz.,
FVC, FEV 0.5, FEF25-75,
MEF25 and PEF increased
minimally until 24 months of age, after that they increased dramatically
with age. FEV0.5/FVC
decreased from baseline until 36 months of age. There was no gender
difference in any of the RVRTC indices except at 36 months where it was
more significant in boys. A multicentric study from Indiana and Ohio
[25] in children from 3 to 149 weeks had also observed that RVRTC
indices were highly correlated with growth (height) of the child while
FEV0.5/FVC decreased with
increasing length. They also did not find any influence of gender on
RVRTC indices except for FEF75,
which was higher in girls [26]. Another study from London has documented
that RVRTC indices increase with age [25].
In conclusion, this prospective birth cohort study
provides reference values for various IPFT indices from neonates to 36
months of age. The median and centile values for boys and girl have been
separately provided. Despite some limitation, the data will be useful as
a reference range for Indian children for TBFVL, RTC, and RVRTC. These
results will serve as normative data for neonates and preschool children
of Indian origin.
Acknowledgments: Satish Thomas, Ritu Dubey
and Rajat Prakash for their contribution in this study.
Contributors: SKK, RL: conceptualized and
designed the study, developed protocol and drafted the manuscript;
PK,SR: enrolled patients, collected and analysed data, reviewed
literature and prepared initial draft of the manuscript; AM: collected
and analysed data, reviewed literature and manuscript preparation; KRJ:
data analysis, reviewed literature and manuscript preparation. All
authors critically revised and approved the final version of the
manuscript.
Funding: The Department of Biotechnology,
Government of India.
Competing interests: None stated.
|
What is Already Known?
• Infant pulmonary
function test (IPFT) can help in the understanding of natural
course and progression of respiratory disease and monitor the
response to therapy in infants and preschool children.
What this Study Adds?
• This study provides the normative data
of IPFT indices in healthy Indian children, and the data can be
used as reference range for infant pulmonary function test in
Indian infant and preschool children.
|
References
1. Vogt B, Falkenberg C, Weiler N, Frerichs I.
Pulmonary function testing in children and infants. Physiol Meas.
2014;35:R59-90.
2. Dinwiddie R. Lung function testing in infants.
Allergol Immunopathol (Madr). 2010;38:337-40.
3. Frey U. Clinical applications of infant lung
function testing: Does it contribute to clinical decision making?
Paediatr Respir Rev. 2001;2:126-30.
4. Baraldi E, Filippone M. Chronic Lung Disease after
Premature Birth. N Engl J Med. 2008;358:743-46.
5. Sly PD, Bush A. From the cradle to the grave: The
early-life origins of chronic obstructive pulmonary disease. Am J Respir
Crit Care Med. 2016;193:1-2.
6. Merkus PJFM, Jongste JCD, Stocks J. Respiratory
function measurements in infants and children. Eur Respir Monogr.
2005;31:166-94.
7. Lai SH, Liao SL, Yao TC, Tsai MH, Hua MC, Yeh KW,
et al. Respiratory function in healthy taiwanese infants: Tidal
breathing analysis, passive mechanics, and tidal forced expiration. PLoS
One. 2015;10:1-13.
8. Kirkby J, Bonner R, Lum S, Bates P, Morgan V,
Strunk RC, et al. Interpretation of pediatric lung function:
Impact of ethnicity. Pediatr Pulmonol. 2013;48:20-6.
9. Koopman M, Zanen P, Kruitwagen CLJJ, Van Der Ent
CK, Arets HGM. Reference values for paediatric pulmonary function
testing: The Utrecht dataset. Respir Med. 2011;105:15-23.
10. Lubchenco LO, Hansman C, Boyd E. Intrauterine
growth in length and head circumference as estimated from live births at
gestational ages from 26 to 42 weeks. Pediatrics.1966; 37:403-8.
11. Frey U, Stocks J, Coates A, Sly P, Bates J.
Specifications for Equipment Used for Infant Pulmonary Function Testing.
ERS/ATS Task Force on Standards for Infant Respiratory Function Testing.
European Respiratory Society/American Thoracic Society. Eur Respir J.
2000;16:731-40.
12. Sly PD, Tepper R, Henschen M, Gappa M, Stocks J.
Tidal Forced Expirations. ERS/ATS Task Force on Standards for Infant
Respiratory Function Testing. European Respiratory Society/American
Thoracic Society. Eur Respir J. 2000;16:741-48.
13. Frey U, Stocks J, Sly P, Bates J, Force T, Ats
ERS. Specification for signal processing and data handling used for
infant pulmonary function testing. Eur Respir J. 2000;16:1016-22.
14. Bates JHT, Schmalisch G, Filbrun D, Stocks J.
Tidal breath analysis for infant pulmonary function testing. Eur Respir
J. 2000;16:1180-92.
15. American Thoracic Society/European Respiratory
Society. Respiratory Function Measurements in Infants: Measurement
Conditions. Am J Respir Crit Care Med. 1995;151:2058-64.
16. Beydon N, Davis SD, Lombardi E, Allen JL, Arets
HGM, Aurora P, et al. An Official American Thoracic
Society/European Respiratory Society Statement: Pulmonary Function
Testing in Preschool Children. Am J Respir Crit Care Med.
2007;175:1304-45.
17. Willis TA, Aspinall BS Nichola MB. Preventing
child obesity: A long-term evaluation of the HENRY approach. Community
Pract. 2013;86:23-7.
18. Cole TJ. The LMS method for constructing
normalized growth standards. Eur J Clin Nutr. 1990;44:45-60.
19. Lodrup-Carlsen KC, Carlsen KH. Lung function in
awake healthy infants: The first five days of life. Eur Respir J.
1993;6:1496-500.
20. Fuchs O, Latzin P, Thamrin C, Stern G,
Frischknecht P, Singer F, et al. Normative data for lung function
and exhaled nitric oxide in unsedated healthy infants. Eur Respir J.
2011;37:1208-16.
21. Lodrup Carlsen KC, Magnus P, Carlsen KH. Lung
function by tidal breathing in awake healthy newborn infants. Eur Respir
J. 1994;7:1660-8.
22. Colin AA, Rao JS, Chen XC, Hunter JM, Hanrahan J,
Hiatt P, et al. Forced expiratory flow in uninfected infants and
children born to HIV-infected mothers. Am J Respir Crit Care Med.
2001;163:865-73.
23. Hoo A, Dezateux C, Hanrahan JP, Cole TJ, Tepper
RS, Stocks J. Sex-specific prediction equations for Vmax (FRC) in
infancy: a multicenter collaborative study. Am J Respir Crit Care Med.
2002;165:1084-92.
24. Henschen M, Stocks J. Assessment of airway
function using partial expiratory flow-volume curves: How reliable are
measurements of maximal expiratory flow at FRC during early infancy? Am
J Respir Crit Care Med. 1999;159:480-6.
25. Hoo AF, Lum SY, Goetz I, Dezateux C, Stocks J.
Influence of jacket placement on respiratory compliance during raised
lung volume measurements in infants. Pediatr Pulmonol. 2001;31:51-8.
26. Jones M, Castile R, Davis S, Kisling J, Filbrun
D, Flucke R, et al. Forced expiratory flows and volumes in
infants: Normative data and lung growth. Am J Respir Crit Care Med.
2000;161:353-9.
|
|
|
 |
|

