|
|
|
Indian Pediatr 2018;55: 55-62 |
 |
Flow Cytometry in Pediatric Malignancies
|
|
Anil Handoo and Tina Dadu
From Centre for Exellence – Flow Cytometry –
Department of Hepatology, Dr BL Kapur Memorial Hospital, New Delhi,
India.
Correspondence to: Dr Anil Handoo, Senior Consultant
Hematology & Director, Laboratory Services, BLK Super Speciality
Hospital, 5 Pusa Road, New Delhi 110 005, India.
Email:
[email protected]
|
|
The utility of flow cytometry as a
useful diagnostic modality for the assessment of hematopoietic neoplasms
has been established beyond doubt. In fact, it is now an integral part
of the diagnosis and classification of various diseases like leukemias
and lymphomas along with molecular studies and cytogenetics.
Prognostication and disease monitoring by flow cytometry is also being
recognized increasingly as one of the important fortes. This is evident
by the number of articles in the published in literature on the minimal
residual disease detection by flow cytometry especially in the last
decade or so. To add to this, ever growing list of utilities in
hematopoietic malignancies, many non-hematopoietic neoplasms can also be
analyzed by flow cytometry. The examples include fluid specimens from
serous cavity effusions and samples from solid tissues like lymph nodes,
reticulo-endothelial tissue, central nervous system tissue, etc. Flow
cytometry technique provides a unique blend of rapidity, high
sensitivity and specificity compared to cyto-morphology and conventional
immunohistochemical staining. It is also remarkable for simultaneous
analysis of more than one marker on the cells. Evaluation of limited
samples such as cerebrospinal fluid or fine needle aspiration samples
makes Flow cytometry a valuable tool. DNA ploidy analysis and assessment
of pediatric non-hematopoietic neoplasms by Flow cytometry has envisaged
the utility vista of this technique. This review is aimed at providing
an insight into the applications of flow cytometry in pediatric
malignancies.
Keywords: Diagnosis, Immunophenotyping,
Lymphoma, Leukemia.
|
|
P
ediatric malignancies, relatively rare and
heterogeneous group of hematological and non-hematological malignancies,
require a multi-modality approach for its diagnostic screening and
classification [1,2], which in turn helps in appropriate therapy and
better outcomes. The need for single cell suspensions in flow cytometry
technique has limited its use for fluid samples, primarily blood and
bone marrow. Therefore, multiparameter flow cytometric
immuno-phenotyping has been primarily used to provide rapid diagnosis
and help classify most of the hematological malignancies, including
pediatric leukemias and lymphomas, where there is blood and/or marrow
involvement, as well as body fluid samples like cerebrospinal fluid,
pleural fluid and peritoneal fluid, etc. Pediatric solid tumors, like
small round cell tumors (SRCT), and nodal lymphomas are increasingly
being subjected to flow cytometry, both for diagnosis and classification
[3]. In addition, flow cytometry is also being used for evaluation of
tumor cell DNA contents and cell cycle analysis [2].
This review is aimed at providing an insight into
utility of flow cytometry in pediatric malignancies for diagnostic
screening, classification, grading, prognostication, therapeutic target
identification and monitoring to name a few. For simplification and
better understanding we shall approach flow cytometry in hematological
disorders followed by flow cytometry in solid malignancies, with focus
on lymph node flow cytometry. Based on the consistency of the available
sample, irrespective of whether the lesion is hematopoietic or
non-hematopoietic, flow cytometry for pediatric malignancies can be
classified into two types: (i) Flow cytometry on liquid/fluid
samples and (ii) Flow cytometry on tissue samples.
Flow Cytometry in Hematological Malignancies
Hematological malignancies in the pediatric age group
are akin to their adult counter parts, albeit with a difference in that
the lymphoid malignancies are more commonly seen and acute lymphoblastic
leukemia happens to be the most common sub-group [4], with diffuse large
B cell lymphoma, Burkitt’s lymphoma and primary mediastinal large B-cell
lymphoma being the other lymphoid malignancies encountered. Mature
lymphomas, especially the low grade lymphomas are extremely uncommon and
their mention is seen only as rare case reports in the literature. Flow
cytometric immunophenotyping forms the cornerstone for accurate
diagnosis and classification of the lymphoid neoplasms. It also forms an
essential part of the armamentarium for the subsequent monitoring by way
of minimal residual disease (MRD) detection. This shall be detailed in
the subsequent sections through this review.
Normal Bone Marrow Differentiation Patterns
It needs to be understood that for identifying
abnormal, a thorough understanding of the normal is essential. Knowledge
of various cluster of differentiation (CD) markers, (common ones used in
routine practice are mentioned in Table I,
immunophenotypic patterns of normal hematopoiesis, especially in bone
marrow samples, and reference patterns for age-related changes is
pivotal for identification of abnormal [5]. Differentiation stages based
on well-defined panels of monoclonal antibodies against specific
antigens helps in delineating various stages of maturation. Pathological
conditions like myelodysplasia or leukemia, show abnormal cells involved
in abnormal differentiation pathways or arrested at a given stage of
differentiation as in acute leukemias. Paediatric bone marrow samples
are relatively unique in that the normal B- cell precursors are in
plenty and mimic lymphoblasts both morphologically and
immuno-phenotypically.
TABLE I Common CD Markers used in Routine Practice
|
Blastic
|
B-Lymphoid |
T-Lymphoid |
Myeloid |
Monocytic |
Erythroid |
Megakaryocytic |
|
nTdt |
CD19 |
CD1a |
cMPO |
CD36 |
CD36 |
CD36 |
|
CD34 |
CD20 |
CD2 |
CD117 |
CD64 |
CD71 |
CD41 |
|
CD38 |
CD10 |
CD3 |
CD13 |
CD14 |
CD235a |
CD42 |
|
HLA- |
lambda |
CD4 |
CD33 |
CD11b |
|
CD61 |
|
DR |
kappa |
CD5 |
CD15 |
|
|
|
|
CD22 (c&s) |
CD7 |
CD16 |
|
|
|
|
cCD79a |
CD8 |
|
|
|
|
|
|
TCR-ab |
|
|
|
|
|
|
TCR-gd |
|
|
|
|
n = Nuclear; c= cytoplasmic; S=Surface; CD45 (Leukocyte Common Antigen): Is the backbone for identification of
various cell populations and is used as the backbone marker for leukemia/lymphoma analysis.
|
Hematogones – Mimic B Lymphoblasts
First described in the 1930s as lymphoid appearing
cells in sternal marrow aspirates, hematogones, by definition,
B-lymphocyte progenitor cells and mature B lymphocytes are normal bone
marrow constituents [6]. They are most prominently seen in pediatric
bone marrows, especially infants and in a variety of diseases in both
children and adults. They are particularly elevated in regenerative bone
marrows, following chemotherapy and bone marrow transplants [6]. In some
instances they may be as high as more than 50% of nucleated cells.
Obviously, because of their morphologic similarity with the blast cells
they closely mimic acute lymphoblastic leukemias. Interestingly, even
immunophenotypically, they closely resemble B- lymphoblasts, however,
the maturation pattern is intact and there is no arrest at a particular
stage. The entire spectrum of antigen expression that defines the normal
evolution of B-lineage precursors is seen on the hematogones as depicted
in the Table II; and also depicted pictorially in
Web Fig. 1. The most common "J-pattern" or the "water-fall
pattern" is shown.
TABLE II Immunological Markers used to Evaluate Hematogones
|
Hematogones |
Mature B Cells |
|
Stage 1 |
Stage 2 |
Stage 3 |
|
|
Tdt |
- |
- |
- |
|
CD34 |
- |
- |
- |
|
CD10 (bright) |
CD10 |
CD10 |
CD10 |
|
CD19 |
CD19 |
CD19 |
CD19 |
|
CD22 (dim) |
CD22 (dim) |
CD22 (dim) |
CD22 |
|
CD38 (bright) |
CD38 (bright) |
CD38 (bright) |
CD38(bright- |
|
CD20 (dim) |
CD20 |
negative) |
|
SIg |
SIg |
CD20 |
|
|
|
SIg |
Use of CD45 (Leukocyte Common Antigen)
Immunophenotypically leukemias can be classified
acute or chronic based on relative positioning of the cells with respect
to mature lymphocyte population on the CD45 side scatter (SSC) dot plot.
A further insight into the data can help further subdivision into
classical AML pattern, classical ALL pattern, CD45 negative pattern
(ALL/AML-M7/AML-M6/Plasma cell pattern), monocytic differentiation
pattern and hematogone pattern provisional groups based on the blast
distribution patterns in the CD45/SSC panel and morphology [7].
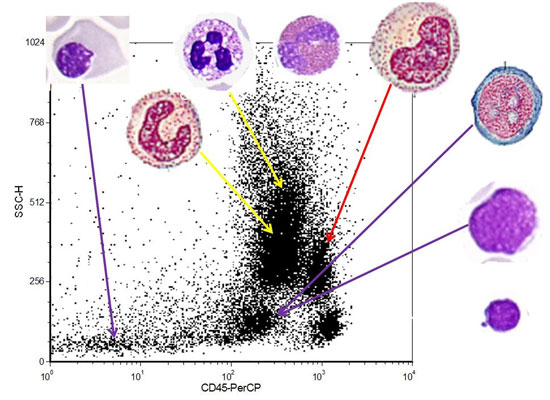
Fig. 1 and Web Fig. 2 shows CD45-SS dot
plots with various patterns. They reveal the blasts of classical AML are
usually seen as a round/oval cluster in the so called blast hole, while
the blasts of a classical B cell ALL distribute horizontally along the
CD45 axis with low side scatter. T cell ALL may have only a minimal to
mild down regulation of CD45 (T-cell ALL pattern), and at times the side
scatter is also not as low as a B cell ALL. CD45 negative pattern is
usually a feature of childhood ALL. It may also be seen in cases of
AML-M7/increased plasma cells and sometimes when there is large amounts
of debris, which happens usually in lyse no-wash technique. A mixed CD45
negative-dim CD45 pattern is usually indicative of an erythroleukemia or
mixed lineage leukemia. A typical APML pattern shows a tear drop pattern
of abnormal cells, starting slightly above the blast hole and going
vertically upwards. But, in majority of the cases it does not touch the
roof. A similar pattern is also seen in cases of chronic myelogenous
leukemia with increased basophils and increased blast cells. The
difference, however, is that the scatter usually starts in the blast
hole and goes vertically up, extending up to the roof of the dot plot.
Hematogone pattern showed cells abutting the lymphocytes with feathering
into the blast region; however, they have a very low side scatter.
Amongst the chronic lymphoproliferative disorders, hairy cell leukemia
(HCL) has a characteristic pattern on the CD45-SS dot plot in that the
neoplastic cells usually are seen on the upper pole of the normal
lymphocyte cluster extending slightly upwards into the monocyte region
(HCL pattern). Most of the other CLPDs are indistinguishable from the
normal lymphocyte population; however, a closer look will make one
identify a minimal down regulation of CD45, so as to be able to identify
a ‘crescent’ of normal lymphocytes (CLPD pattern) [7].
 |
|
Fig. 1 CD45-Side Scatter patterns with
relative placement of various cells.
|
Acute Leukemia Diagnosis
While morphology continues to be the cornerstone for
acute leukemia diagnosis, and presence of >20% blasts in the blood and
bone marrow is suggestive of the disease, immunophenotypic features of
blasts cells are pivotal in defining the stage of maturation arrest of
the blast population not only within the B- and T-lymphoid lineages but
for the neutrophilic, monocytic, megakaryocytic or erythroid lineages as
well. As mentioned earlier, CD45 continues to be the anchoring marker to
identify the presence of blast cells. The blast cells are usually dim
CD45 compared to normal lymphocytes. Peculiarly, pediatric B-cell acute
lymphoblastic leukemias may be dim to completely negative for CD45.
Therefore, it is essential to ascertain the expression of other markers
to identify the abnormal blasts. A combination of CD19 (Pan B cell
marker) along with blastic markers, CD34, Tdt and HLA DR, help in
delineating the blasts, in majority of the patients. CD10, CD20, CD15
are other markers which are useful in identifying B lymphoblasts [8]. An
important sub-type, which is CD10 negative and CD15 positive has been
seen to be associated with mixed lineage leukemia (MLL) gene
abnormalities.
T cell acute lymphoblastic leukemias (T cell ALLs) on
the other hand have relatively brighter CD45, sometimes as bright as
normal T cells. Classically, T cell ALLs are surface CD3 negative and
cytoplasmic CD3 positive; In addition most of them are usually CD4 and
CD8 double negative or double positive. They can also be restricted to
either CD4 or CD8, when they are classified as medullary T cell ALLs. A
special mention about early precursor T cell ALL (ETP ALL), which has a
relatively poorer prognosis has characteristic phenotype of CD1a(-),
CD8(-), CD5(-) (dim), and positivity for 1 or more stem cell or myeloid
antigens [9].
Acute Myeloid leukemias (AML), though relatively
rarer than ALL in the pediatric age group, is usually diagnosed based on
morphology and molecular and cytogenetic features. Flow cytometry,
however, still has a role in sub-classification and in some instances
may be extremely important for diagnosis, like acute myeloid leukemia
with minimal differentiation as well as megakaryoblastic leukemias [10].
While Table I details various markers that are usually
used for sub-typing AMLs, cytoplasmic myeloperoxidase is the most
important marker that helps in assigning myeloid lineage.
Bi-phenotypic acute leukemia (BAL) is a very rare
disease possibly arising from a hemopoietic pluripotent stem cells, are
almost exclusively defined by Immunophenotyping. In fact, the scoring
system proposed by the European Group for the Immunological
classification of Leukemias (EGIL), was based solely on flow cytometric
immunophenotyping, till World Health Organization (WHO) defined this
entity as mixed phenotype acute leukemias (MPAL) in 2008. While the
specificity for T-lymphoid and myeloid is based on cytoplasmic CD3 and
cytoplasmic myeloperoxidase (MPO) antigens, respectively, the latter
shown by either flow cytometry (FCM) or cytochemistry and/or clear
evidence of monocytic differentiation, since there is no specific single
antigen for B cells, lineage assignment here relies on the strong
expression of CD19 together with another B cell-associated marker or, in
cases with weak CD19, on the expression of at least 3 B-lineage markers
[11]. Web Fig. 3 shows few common acute leukemia
phenotypes.
Lymphoma Diagnosis
Primarily based on morphologic assessment of the
tissues involved with the disease is done with immunohistochemistry as
an adjunct in the diagnostic algorithm. Diagnosis of lymphomas using
flow cytometry has mainly been used for non-Hodgkin’s lymphoma, though
literature does describe its use in Hodgkin’s lymphoma. Hitherto, flow
cytometry was primarily used for diagnosis of lymphomas on fluid samples
should they be available for analysis based on their involvement. In the
pediatric age group, low grade lymphomas are extremely rare and the
usual lymphomas seen include diffuse large B cell lymphoma, Burkitt’s
lymphoma and primary mediastinal large B cell lymphoma. T cell
lymphomas, seen include hepato-splenic T cell lymphoma and rarely,
peripheral T cell lymphoma (not otherwise specified). Utility of flow
cytometry has been dealt with in the subsequent section on flow
cytometry on lymph nodes.
Minimal Residual Disease (MRD) Detection Using Flow
Cytometry in Pediatric Leukemias
Minimal residual disease (MRD) is defined as disease
undetectable by morphologic examination. MRD is gaining importance
nowadays both for therapy efficacy follow up and relapse risk
estimation. Flow cytometric detection of MRD is based on identification
of leukemic cells and their differentiation form normal, healthy cells
by expressions of aberrant antigens or other phenotypic characteristics,
called leukemia associated immuno-phenotypes (LAIPs). The advantages
that FCM presents vis-a-vis other techniques is easy
availability, rapid, convenient, and generally applicable technique for
detecting MRD.
Dario Campana and Elane Coustan Smith were the
pioneers in this field, especially in the detection of MRD in pediatric
B cell ALLs. They not only demonstrated the effectiveness of flow
cytometry in this area, but also helped in getting the therapy protocols
based on the MRD analysis [12]. A cut-off of 0.01% and below is
suggestive of the absence of the disease. Advances and upgradation of
the technology in the area of flow cytometry including having additional
lasers, newer antibodies, more fluorochromes and possibility of
evaluation of more than 8 colors and adoption of bulk lysis method, the
limit of detection has gone up significantly from 0.01% to now 0.001%.
However, in centres with resource constraints, where 3-4-colour flow
cytometers are still in use, MRD detection by ‘MRD lite’, using only 3
antibodies (CD19, CD34 and CD10) may be a useful tool to detect MRD in
the bone marrow on Day 19 of induction chemotherapy, up to a detection
limit of 0.01%. The MRD lite analysis is based on the fact that on Day
19, not >0.01% precursor B cells (hematogones) are present in the bone
marrow. And thus presence of >0.01% CD19 positive B cells which
co-express either CD34 and/or CD10 would be residual blasts, and not
hematogones (which are also CD19+ with CD34 and/or CD10) [13,14]. This
methodology has its disadvantages as (a) it cannot be used to
evaluate MRD on Day 28-33 after completion of induction therapy which
has better prognostication than MRD done on day 19; (b) not using
LAIPs to differentiate residual blasts from precursor B cells may give
false positive results, and (c) the limit of detection is 0.01%,
which is one log lower than the one with advanced flow cytometry.
Web Fig. 4 shows scatter plots demonstrating the MRD in a case
of B and T ALL.
Flow Cytometry on Lymph Nodes
Lymphadenopathy is a common finding in children. Most
of the times it is benign; however, it is a worrisome situation and
needs diagnostic workup. The diagnosis of disease in a lymph node has
always been based on morphology, be it a lymphoma, leukemia, metastasis
or even reactive. Flow cytometry can provide a faster diagnosis with the
same material provided for morphology, the material being an excised
lymph node or fine-needle aspirate (FNA) from lymph node. While the
morphology remains the cornerstone for diagnosis of hemato-lymphoid
neoplasia, recent update of the WHO Classification of Tumours of
Haematopoietic and Lymphoid Tissues (2016), has laid a significant
emphasis on various mutations and genetic signatures. Despite this,
immunophenotype continues to be an integral component for
sub-classification, especially for lymphoid neoplasia. Immunophenotyping
can be done by both, immuno-histochemistry (IHC) (done on tissue
section) and flow cytometry (FCM) (done on cells extracted from tissue),
with FCM clearly scoring over IHC on may counts. Faster turnaround time,
pick-up of immuno-phenotypically discrete cell populations, evaluating
co-expression of various markers by multi-parameter FCM, quantitation of
antigen expression based on mean/median fluorescence intensity and even
grading of lymphomas by S-phase fraction analysis are some of the
salient advantages of FCM versus IHC [15].
Lymphomas are the third most common childhood
malignancies after acute leukemias and brain tumors, constituting
10-12% of childhood cancers [16]. Burkitts Lymphoma which is a high
grade aggressive B cell Non Hodgkins Lymphoma (NHL) and considered to be
a medical emergency, is the most common NHL in children and adolescents
and accounts for around ~40% of NHL’s in those under the age of twenty
[17]. A faster diagnosis by flow cytometry in this situation can be life
saving. Flow cytometry utilizes a panel of anibodies for
immuno-phenotyping, based on which a diagnosis is made. It serves a
variety of roles in the field of diagnosis including:
Acute Lymphoblastic Lymphoma: Flow cytometry is
magical in picking up blastic population, by clearly separating abnormal
blasts from the rest of the population using CD45. These abnormal blasts
can then be further classified based on the array of antibodies used.
Example: 5-year-old boy presented with
mediastinal mass. Tissue Biopsy done showed dim CD45 positive blasts,
expressing cytoplasmic CD3, dim to negative CD3, very bright CD7, dim
CD2, and dual negative CD4 and CD8, consistent with a diagnosis of T
cell lymphoblastic lymphoma (Web Fig. 5)
Lymphomas
(a) Diagnosis: Conventionally,
diagnosis of lymphomas has been based on biopsy sections, both
excised tissue as well as needle biopsies. Supplementation with IHC
on these tissues has helped confirm, classify and sub-categorize,
which in turn has ensured appropriate therapy protocols. While
diagnosis has been made in majority of the patients based on the
above mentioned procedures, few of the cases elude diagnosis for the
fact that the involvement is subtle or have a considerable overlap
with non-neoplastic entities. Immuno-phenotypic diagnosis for most
of the hemato-lymphoid neoplasia requires an array of markers.
Lymphomas, especially, non-Hodgkin’s lymphoma, provide a unique
opportunity to base the diagnosis on clonality assessment. The
demonstration of clonality, for B-cells is done by using kappa and
lambda light chains, best done on FCM since one is able to select
discrete populations and look at the expression of light chains on
them, unlike IHC, wherein a lot of background staining is noted on
the sections, making it difficult for interpretation. T-cell
clonality is relatively difficult both on tissue by IHC as well as
by FCM and the diagnosis is primarily based on immuno-phenotypic
aberrancies on the T cells. In addition, using antibodies against
the variable region of T cell receptor antigen – beta (TCR- ub),
clonal restriction to any of the 24 sub families of TCR-ub
can be assessed by FCM [15]. It also needs to be noted that in
today’s era of personalized medicine and targeted therapies, FCM
helps in identification of various antigens for which targeted
therapies are available, e.g. rituximab as anti CD20,
alemtuzumab as anti CD52, daratumumab as anti CD38.
(b) Classification: Classifying
lymphomas is needed to tailor therapy as per the immunophenotypic
sub-groups. Application of a repertoire of antibodies helps in
classification. While these can be applied on the tissue, FCM has a
unique advantage of using minimal sample for applying them and
easier interpretation since expression of various markers is
ascertained on discrete cell populations. This becomes important
when one is looking for expression of antigens not native to the
population of interest [15]. For example, expression of CD5 (a T
cell antigen) on B cells, i.e. CD5+ B cells. An algorithmic
approach to immuno-phenotypic diagnosis of NHL is depicted in the
Fig. 2.
(c) Estimation of S-Phase Fraction:
Historically, morphological grading of lymphomas has been done
morphologically and by Ki-67 immunostaining on the tissues.
Morphologists have long been arguing on how, flow cytometry is in
adept to provide this valuable information, which has both
prognostic and therapeutic implications. However, the possibility of
staining DNA and quantifying the same at various stages in cell
cycle has made this easily available. Flow cytometry, helps in
provision of reliable and more reproducible count of grading by
‘S-Phase faction’. More importantly, the same is assessed on
selected cell populations of interest [15]. An S-Phase fraction of
<10% has been seen in low grade lymphomas, whereas >15% is seen in
high grade lymphomas. An example of Burkitts lymphoma expressing
CD19, CD20, CD10 and lambda restriction with High S-phase fraction
is demonstrated in Web Fig. 6.
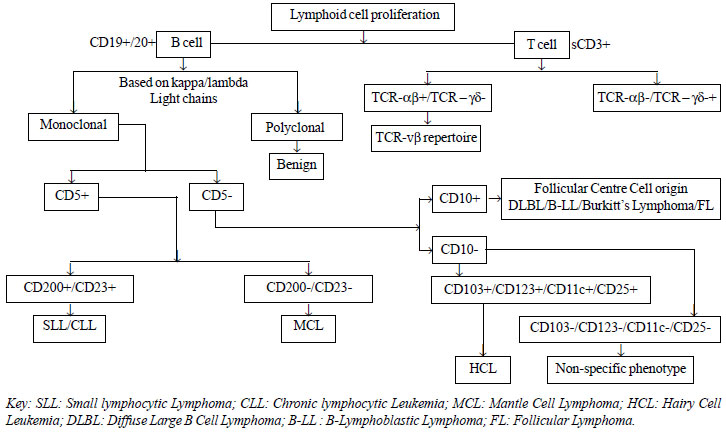 |
|
Fig. 2 Algorithm for immunophenotypic classification of
NHL.
|
Flow cytometry, thus, is a valuable technique for
immunophenotypic analysis of lymphomas, especially the NHLs. Researchers
have also demonstrated its ability to characterize Hodgkin’s Lymphoma as
well. A few disadvantages, though, include requirement of fresh unfixed
tissue and immediate processing as well as interpretative issues in some
cases of T-cell rich B cell lymphoma.
DNA Ploidy and Cell Cycle Analysis
Flow cytometry is a rapid and reliable method for
measuring nuclear DNA content [18]. Measurement of DNA content of
individual cells helps provide information about their ploidy, which is
of relevance in some neoplastic disorders like ALL, breast tumors,
lymphomas, etc. DNA content of the cells is measured by staining with a
fluorescent dye that binds to DNA ensuring reflection of an accurate
amount of DNA present [18]. Propidium iodide (PI), is one of the most
widely used dyes for such purposes. DRAQ5, FxCycle, bis-benzimadazole,
Hoechst 33342, etc are other dyes used which help to observe a DNA
histogram in viable cells without permeabilisation, unlike dyes like PI
[18].
Flow Cytometry of Non-hematopoietic Neoplasms
Flow cytometry is not used routinely in the diagnosis
or follow-up of non-hematopoietic neoplasms; however, many
non-hematopoietic neoplasms and tissues are amenable to flow cytometric
analysis, especially serous cavity effusions and limited fine-needle
aspirate (FNA) or cerebrospinal fluid (CSF) samples [19]. It is possible
to differentiate various non-hematopoietic malignancies with use of
certain markers like EpCAM (Ber-EP4, CD326, and MOC31), CD45, CD56,
CD71, CD81, CD9, MyoD1, Myogenin, CD99 and CD271. For instance, most
carcinomas would be EpCAM+, CD45–, CD14–, while neuroblastomas would
have the following profile: CD56hi, GD2+, CD81+, CD9+, CD45–. Rhabdomyo-sarcomas
on the other hand would also express MYOD1+, myogenin+ [2,19]. In fact
based on the following markers CD45, CD56, CD90, EpCAM, CD34, Myogenin
and MyoD1, an algorithm for differentiating various small round cell
tumours can be followed. Details of markers with their relative
expressions is given in the Table III. An algorithmic
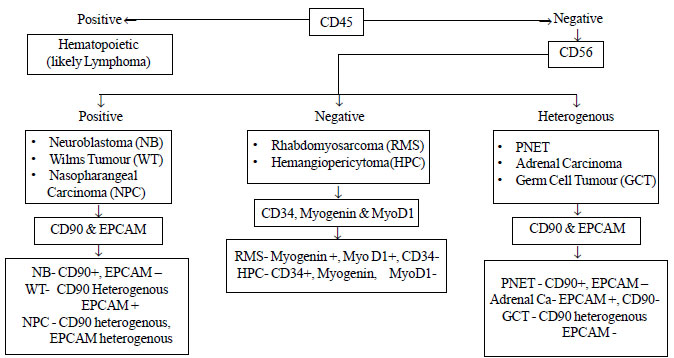
approach to the diagnosis is also provided in the Fig.3.
CD45 would clearly help differentiate hematopoietic (CD45+) versus non
hematopoietic malignancies (CD45-). All the non-hemopoeitic malignancies
can then be subdivided into three categories based on CD56 expression. A
strong expression of CD56 is seen in Neuroblastoma, Wilms’ tumour and
Nasopharygngeal carcinoma. These can be further characterised based on
the expressions of EpCAM and CD90. Neuroblastoma is CD90 positive and is
negative for EpCAM-, Wilms tumour is strongly positive for EpCAM with a
heterogenous expression of CD90, whereas nasopharygngeal carcinoma shows
a heterogenous expression for both CD90 and EpCAM. Rhabdomyosarcoma and
hemangiopericytoma are negative for CD56, and based on myogenin and
myo-D1 positivity classified as rhabdomyosarcoma and CD34 positivity as
hemangiopercytoma. A heterogenous expression of CD56 suggests the
possibility of primitive neuroectodermal tumour (PNET), adrenal
carcinoma or germ cell tumour (GCT). These can again be differentiated
based on CD90 and EpCAM. PNET is CD90 positive and is negative for EpCAM-,
while adrenal carcinoma has an inverse expression of these markers,
being positive for EpCAM and negative for CD90. GCT is also EpCAM
negative; however, it shows heterogenous expression for CD90. As is
obvious from the above discussion, pediatric small round cell tumors,
would clearly be amenable to accurate diagnosis using flow cytometry
within a reasonably reduced time frame.
TABLE III Immunophenotypic Markers for Small Round Cell Tumors [2]
|
CD56
|
CD90
|
CD99
|
CD9
|
CD81
|
MyoD1
|
Myogenin
|
EPCAM
|
CD271
|
CD34
|
|
Neuroblastoma
|
+
|
+
|
_
|
+
|
+
|
_
|
_
|
_
|
_
|
_
|
|
PNET
|
H
|
+
|
+
|
+
|
+
|
_
|
_
|
_
|
+
|
_
|
|
Rhabdomyosarcoma
|
_
|
+
|
_
|
H
|
H
|
+
|
+
|
_
|
_
|
_
|
|
Wilms tumour
|
+
|
H
|
_
|
H
|
H
|
_
|
_
|
H
|
H
|
_
|
|
Adrenal carcinoma
|
+
|
H
|
_
|
H
|
H
|
_
|
_
|
+
|
_
|
_
|
|
Nasopharyngeal carcinoma
|
+
|
H
|
_
|
H
|
H
|
_
|
_
|
+
|
_
|
_
|
|
Germ cell tumour
|
+
|
H
|
_
|
H
|
DIM +
|
_
|
_
|
_
|
_
|
_
|
|
Hemangiopericytoma
|
_
|
_
|
_
|
_
|
_
|
_
|
_
|
_
|
_
|
+ |
+ positive, – negative, H heterogeneous.
|
 |
|
Fig. 3 Algorithm for diagnosing
small round cell tumor by flow cytometry.
|
Summary
Pediatric malignancies comprise a heterogenous group
of disorders, which need a multi-modality approach for diagnosis. Flow
cytometry is increasingly being acknowledged as a valuable tool in the
diagnostic algorithm. This is especially so in the hemato-oncological
disorders like acute leukemias. Flow cytometry is also showing its
imprint in grading and prognostication of various disorders, DNA ploidy
and minimal residual disease detection being the prime examples of the
same. Tissue samples and many non-hematopoietic malignancies are also
increasingly subjected to flow cytometry for appropriate diagnosis and
classification. Use of flow cytometry in conjunction with other adjunct
modalities like molecular and cytogenetic studies is ensuring an
accurate diagnostic and prognostication realm.
References
1. Arora RS, Eden TOB , Kapoor G. Epidemiology of
child-hood cancer in India. Indian J Cancer. 2009;46:264-73.
2. Ferreira-Facio CS, Milito C, Botafogo V, Fontana
M, Thiago LS, Oliveria E, et al. Contribution of multiparameter
flow cytometry immunophenotyping to the diagnostic screening and
classification of pediatric cancer. PLoS ONE. 2013;8: e55534.
3. Pillai V, Dorfman DM. Flow cytometry of non-hema-topoietic
neoplasms. Acta Cytologica. 2016;60:336-43.
4. Li J, Wertheim G, Paessler M, Pillai V. Flow
Cytometry in pediatric hematopoietic malignancies. Clin Lab Med.
2017;37:879-93.
5. Arnoulet C, Be´ne´ MC, Durrieu F, Feuillard J,
Fossat C, Husson B, et al. Four- and five-color flow cytometry
analysis of leukocyte differentiation pathways in normal bone marrow: A
reference document based on a systematic approach by the GTLLF and GEIL.
Cytometry Part B:Clin Cytometry. 2010;78B:3-10.
6. Mckenna RW, Asplund SL, Kroft SH. Immunophenotypic
analysis of hematogones (B-lymphocyte precursors) and neoplastic
lymphoblasts by 4-color flow cytometry. Leuk Lymphoma. 2004;45:277-85.
7. Handoo A, Dadu T, Rangan A, Bachchas V, Sartor M,
Choudhary DR, et al. CD45-side scatter dot plot in malignant
hematological flow cytometry – scanner view for prediction of
immunophenotypic diagnosis/lineage assignment and an optimizing tool for
cost effective flow cytometry. Int J Lab Hemat. 2012;34:50 (Abstract).
8. Wang XM. Advances and issues in flow cytometric
detection of immunophenotypic changes and genomic rearrangements in
acute pediatric leukemia. Transl Pediatr. 2014;3:149-55.
9. Jain N, Lamb AV, O’Brien S, Ravandi F, Konopleva
M, Jabbour E, et al. Early T-cell precursor acute lymphoblastic
leukemia/lymphoma (ETP-ALL/LBL) in adolescents and adults: a high-risk
subtype. Blood. 2016;127:1863-9.
10. Craig FE, Foon KA. Flow cytometric
immunophenotyping for hematologic neoplasms. Blood. 2008;111:3941-67.
11. Borowitz MJ, Bene MC, Harris NL, Porwit A, Matutes E.
Acute leukemias of ambiguous lineage. In: Swerdlow SH, Campo E,
Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J Vardiman JW,
editors. WHO Classification of Tumours of Haematopoietic and Lymphoid
Tissues. 4th ed. Lyon, France: IARC Press; 2008. p. 150-5.
12. Campana D, Coustan-Smith E. Detection of minimal
residual disease in acute leukemia by flow cytometry. Cytometry.
1999;38:139-52.
13. Coustan-Smith E, Ribeiro RC, Stow P, Zhou Y, Pui
CH, Rivera GK, et al. A simplified flow cytometric assay
identifies children with acute lymphoblastic leukemia who have a
superior clinical outcome. Blood. 2006;108:97-102.
14. Chatterjee T, Somasundaram V. Flow cytometric
detection of minimal residual disease in B-Lineage acute lymphoblastic
leukemia by using "MRD lite" panel. Med J Armed Forces India.
2017;73:54-7.
15. Braylan RC. Impact of flow cytometry on diagnosis
and characterization of lymphomas, chronic lymphoproli-ferative
disorders and plasma cell neoplasias. Cytometry A. 2004;58:57-61.
16. Consensus Document for Management of Pediatric
Lymphomas and Solid Tumors – Prepared as an outcome of the
ICMR-sub-committee on paediatric lymphomas and solid tumors. ICMR
2017:29. Available at URL:
http://www.icmr.nic.in/guide/cancer/PEDIATRIC%20
LYMPHOMAS%20AND%20SOLID%20TUMORS%20 final%20pdf.pdf. Accessed
October 09, 2017.
17. Miles RR, Arnold S, Cairo MS. Risk factors and
treatment of childhood and adolescent Burkitt lymphoma/leukaemia. Br J
Haematol. 2012;156:730-43.
18. Darzynkiewicz Z, Halicka HD, Zhao H. Analysis of
cellular DNA content by flow and laser scanning cytometry. Adv Exp Med
Biol. 2010;676:137-47.
19. Pillai V, Dorfman DM. Flow cytometry of nonhe-matopoietic
neoplasms. Acta Cytologica. 2016;60: 336-43.
|
|
|
 |
|

