|
|
|
Indian Pediatr 2017;54: 53-54 |
 |
Blue Rubber Bleb Nevus
Syndrome: Promising Response To Sirolimus
|
|
Canan Akyuz, Hilal Susam-Sen and Burca Aydin
From Department of Pediatric Oncology, Hacettepe
University, Cancer Institute, 06100 Ankara-Turkey.
Correspondence to: Dr Burca Aydin, Department of
Pediatric Oncology, Hacettepe University, Cancer Institute, 06100
Ankara-Turkey.
Email: [email protected]
Received: January 07, 2016;
Initial review: March 07, 2016;
Accepted: November 11, 2016 .
|
Background: Blue rubber bleb nevus syndrome is a rare disease
involving venous malformations. Case characteristics: We present
a 6-year-old female with the syndrome, and consumptive coagulopathy.Intervention/Outcome:
After management with sirolimus, symptoms resolved. Message:
Sirolimus may be a valuable option for reducing bleeding complications
and cosmetic sequelae for the patients with this syndrome.
Keywords: Hemangioma, Treatment, Vascular malformations.
|
|
B
lue rubber bleb nevus syndrome is a rare disease
characterized by venous malformations on the skin, soft tissue and
visceral organs, predominantly gastro-intestinal (GI) tract [1]. Skin
lesions are multiple soft vascular lesions, which may be papular,
nodular or pedunculated, and red or deep blue in color [2]. Medical
treatment with steroids, interferon and octreotide has been reported
with some success but in most reported patients lesions regrow [3].
Sirolimus is an inhibitor of mammalian target of
rapamycin (mTOR) and has been recently used for vascular anomalies with
considerable success [4,5]. We report a patient with this syndrome who
was unresponsive to steroids and interferon, and was treated
successfully with sirolimus.
Case Report
A 6-year-old female was admitted to our hospital with
multiple skin lesions and anemia. The lesions appeared at the age of six
months and increased in size and number. Oral steroids were given at a
dose of 1 mg/kg/day for few months and sclerotherapy was performed for
her largest and painful lesion in the left cervical region
without any success. At the age of five she had GI bleeding and
colonoscopy showed multiple vascular ectasias and venous malformations.
She had been transfused many times in previous year. On admission, she
was pale with widespread small variable-sized bluish papules and large
vascular masses on the face, mouth, trunk, arms, legs and fingers were
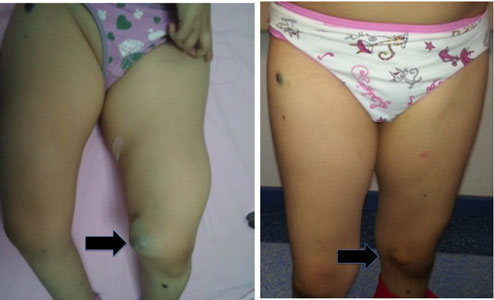
noted (Fig. 1a). She had pain in her left knee where the
largest vascular lesion was located. Blood tests revealed hemoglobin
6.1g/dL, white blood cell count 8100/mm³, platelets count 77,000/mm³,
unconjugated bilirubin 1.6 mg/dL, fibrinogen 104 mg/dL, and D-dimer
>40mg/dL. Acanthocytes and schistocytes were noted on perepheral blood
smear. The patient was diagnosed as Blue rubber bleb nevus syndrome with
typical clinical findings with microangiopathic hemolytic anemia and
active consumptive coagulopathy. She also had mild GI bleeding shown
with microscopic blood from rectum and fresh frozen plasma and packed
red cell transfusions were given. At the end of 11th day, due to no
response to medical treatment for anemia and consumptive coagulopathy,
sirolimus was started orally at a dose of 1,6 mg/m 2/day.
Serum sirolimus level was measured weekly and dose adjusted to maintain
the therapeutic level between 5-12 ng/mL. Sirolimus level was stabilised
at the dose of 2mg/m2/day,
but during follow-up daily dose had to be adjusted occasionally. The
outcome was assessed by monitoring the reduction in size and color of
the lesions. Improvement was noted at day 7, as the size and numbers of
lesions were decreased and hematologic findings dramatically improved
over days and achieved normal (Fig. 1b). On the 15th
day of sirolimus, supportive medications were stopped, her pain resolved
and she started walking. The drug was well tolerated and no side effects
were seen. She was treated with sirolimus for 17 months. Cutaneous
lesions continued to regress during therapy, and no further GI bleeding
or anemia was observed. She has been now off-therapy for 4 months
without any microscopic blood in stool and normal hemaglobin levels. Her
cutaneous lesions are stable and have not progressed since cessation of
treatment.
 |
| (a) |
(b) |
|
Fig. 1 Vascular mass on left knee (a);
regression of the vascular mass; after eight week of sirolimus
treatment (b).
|
Discussion
Blue rubber blub nevus syndrome is heterogeneous in
phenotypic expression. The skin lesions arise at birth or early infancy
mostly in limbs, trunk and face and vary from bluish black macules or
papules to large venous malformations. The size and number of skin
lesions tend to increase with age. Visceral lesions can be seen in any
sites in the body, but small intestine is the most commonly involved
organ. The lesions are fragile and bleed easily. Occult iron deficiency
anemia or massive hemorrhage can occur. Bleeding occasionally cause
platelet entrapment and consumptive coagulopathy, as in our patient.
Cutaneous lesions usually do not require treatment, unless they cause
cosmetic or functional problems. The treatment of GI lesions depends on
the intensity of bleeding. Occult bleeding and anemia might only need
iron supplementation. Massive GI bleeding is the most serious
complication of vascular lesions [2,6].
No curative therapy is available for Blue rubber bleb
nevus syndrome. Medical treatment including steroids, propranolol and
interferon alpha had been reported with variable effect [3]. In almost
all cases, lesions regrew to their pretreatment sizes after the
treatment was stopped [3]. Our patient demonstrated no response to
steroids and interferon previously, however, after sirolimus, was
started Hb level stabilized, GI bleeding decreased, and consumptive
coagulopathy resolved. No side effect including hyperlipidemia,
mucositis, diarrhea, neutropenia, headache, peripheral edema or
respiratory distress were observed [4].
Sirolimus has been increasingly used for vascular and
lymphatic anomalies and kaposiform hemangioendo-thelioma [3-5]. Hammill,
et al. [4] reported 6 cases of venous and lymphatic malformations
successfully treated with Sirolimus. We previously reported another
patient with giant lymphatic malformation in tongue showing near-total
regression after sirolimus [7]. Sirolimus has not yet been demonstrated
in clinical trial but is a promising new therapy for a condition not
previously medically managed well. Sirolimus should be considered as
first-line treatment for treating GI and cutaneous vascular
malformations in Blue rubber bleb nevus syndrome.
Contributors: CA: contributed the planning
treatment and follow-ups of the patient, reviewed and revised the
manuscript, and approved the final manuscript as submitted; HS:
contributed the follow-ups of the patient, drafted the initial
manuscript, and approved the final manuscript; BA: contributed the
planning treatment and follow-ups of the patient, reviewed and revised
the manuscript, and approved the final manuscript as submitted.
Funding: None; Competing interest: None
stated.
References
1. Bean WB. Vascular Spiders and Related Lesions of
the Skin. Springfield, IL: Charles C Thomas. 1958:178-85.
2. McKusick VA. Blue rubber bleb nevus (Bean
syndrome). In: McKusick VA, ed. Mendelian Inheritance in Man.
11th ed. Baltimore: John Hopkins University Press; 1994: 212-3.
3. Yuksekkaya H, Ozbek O, Keser M, Toy H. Blue rubber
bleb nevus syndrome: successful treatment with sirolimus. Pediatrics.
2012;129:e1080-4.
4. Hammill AM, Wentzel M, Gupta A, Nelson S, Lucky
A, Elluru R, et al. Sirolimus for the treatment of complicated
vascular anomalies in children. Pediatr Blood Cancer. 2011;57:1018-24.
5. Blatt J, Stavas J, Moats-Staats B, Woosley
J, Morrell DS. Treatment of childhood kaposiform hemangio-endothelioma
with sirolimus. Pediatr Blood Cancer. 2010;55:1396-8.
6. Nahm WK, Moise S, Eichenfield LF, Paller AS, Nathanson
L, Malicki DM, et al. Venous malformations in blue rubber bleb
nevus syndrome: variable onset of presentation. J Am Acad Dermatol.
2004;50:101-6.
7. Akyüz C, Ataº E, Varan A. Treatment of a tongue
lymphangioma with sirolimus after failure of surgical resection and
propranolol. Pediatr Blood Cancer. 2014;61:931-2.
|
|
|
 |
|

