|
|
|
Indian Pediatr 2017;54: 33-36 |
 |
Neonatal Diabetes: A
Case Series
|
|
Ramaswamy Ganesh, Natarajan Suresh, Thiruvengadam
Vasanthi and *KG Ravikumar
From Departments of Pediatrics and *Endocrinology,
Kanchi Kamakoti CHILDS Trust Hospital and The CHILDS Trust Medical
Research Foundation, Chennai, Tamil Nadu, India.
Correspondence to: Dr Ramaswamy Ganesh, Consultant
Pediatrician, Kanchi Kamakoti CHILDS Trust Hospital, Chennai 600 034,
India.
Email:
[email protected]
Received: October 26, 2015;
Initial review: January 11, 2016;
Accepted: September 02, 2016.
Published online: November 05, 2016.
PII:S097475591600020
|
Background: Neonatal diabetes
mellitusis a rare disorder with an incidence of 1 in 2,60,000 live
births. Methods: Retrospective analysis of clinical and genetic
profile of children admitted with neonatal diabetes mellitus in a
tertiary-care hospital in Chennai, India over 11 years. Results:
Ten children were diagnosed with neonatal diabetes of whom 9 had
permanent neonatal diabetes mellitus. The age range at onset was from 3
days- 5 months. Of the 9 children, KCNJ11 gene mutation was
positive in one, and ABCC 8 and INS gene mutation in two
children each. Children with KCNJ11 and ABCC 8 gene
mutations were switched over to oral sulfonyl urea therapy.
Conclusion: Few genotypes causing NDM can be managed effectively
with oral sulfonyl ureas.
Keywords: Diabetes mellitus, Genetics, Permanent, Transient.
|
|
M
onogenic diabetes results from the inheritance of
a mutation or mutations in a single gene [1], and accounts for 1-5% of
all childhood diabetes [2]. Neonatal diabetes mellitus (NDM) and
maturity-onset diabetes of the young (MODY) are the two main forms of
monogenic diabetes. NDM first occurs in newborns and young infants; MODY
usually first occurs in children or adolescents but may be mild and not
detected until adulthood. Most patients with monogenic diabetes are
incorrectly diagnosed as either type 1 or type 2 diabetes. Identifying
this entity correctly not only helps to initiate appropriate treatment
but also helps us to explain the other associated clinical features and
offer genetic counseling to the family for subsequent pregnancies [3].
An earlier study from Chennai [4] had reported 28 children with
neonatal diabetes (0.05%) and 12 children with diabetes onset between 6
months to 1 year of age out of 506 diabetic children registered in their
institute. The common gene mutations reported in their series were
ABCC8 followed with EIF2AK3 and KCNJ11.
We describe the clinical features and follow-up of
children with neonatal diabetes from an urban children’s hospital in
Chennai, India.
Methods
A retrospective analysis of case records of children
admitted with neonatal diabetes mellitus in the Department of Pediatrics
and Endocrinology of Kanchi Kamakoti CHILDS Trust hospital, Chennai from
January 2004 to December 2014 were analyzed. The study was approved by
the institutional review board. A diagnosis of neonatal diabetes
mellitus was established in infants who had their onset of diabetes
within the first 6 months of life and presented with features of
polyuria, polydipsia, weight loss, DKA and had their fasting blood sugar
>126 mg/dL with HbA1C >6.5%. The case records of infants with neonatal
diabetes mellitus were analyzed for birth weight, the age at onset of
symptoms, the clinical features, laboratory investigations (FBS, HbA1C
values), Genetic mutation testing results, treatment and follow-up
details. We collected 3 mL of whole blood in EDTA tube from the proband
and their parents, and sent it to Royal Devon and Exeter NHS Foundation
Trust laboratory, Exeter, UK for genetic analysis. Molecular genetic
testing included gene sequencing by PCR technique. All infants were
treated with subcutaneous insulin at 05 -0.8 U/kg/day and were followed
up.
Results
During the study period, a total of 137 children were
diagnosed as Type 1 diabetes mellitus as per WHO diagnostic criteria and
10 (5 boys) were diagnosed as neonatal diabetes mellitus. The age range
at onset was from 3 days to 160 days. Six children were born to parents
with consanguineous marriage and none had history of diabetes in their
first degree relatives. All were born at term and six were born with a
birth weight <2.5 kg. Diabetic ketoacidosis was the mode of presentation
in 3 (30%) children (INS,EIF2AK3 and NEUROD1 gene).
Glutamic acid decarboxylase and islet cell autoantibodies were negative
in all children. The mean blood sugar was 499 mg/dL. Of the 10 children,
one child had transient neonatal diabetes mellitus and nine had
permanent neonatal diabetes mellitus. The child with transient neonatal
diabetes presented with hyperglycemia on D3 of life, required insulin
for 5 months, and mutation analysis revealed complete loss of
methylation on chromosome 6 q24. She is off insulin and at her 16 month
follow-up, she is growing well. Of the nine children with permanent
neonatal diabetes mellitus, KCNJ11 gene mutation was positive in
1, ABCC 8 gene and INS gene mutation in 2 each, PDX1
gene mutation in 1, NEURO D1 mutation in 1, EIF2AK3
mutation in 1 and SLC19A2 gene mutation in 1 child. Children with
KCNJ11 gene mutation and ABCC 8 gene mutation were treated
with oral sulfonyl urea and others were treated with Insulin. On follow
up, the child with Wolcott Rallison Syndrome died and other patients are
growing well without problems. The details are shown in Table
I.
TABLE 1 Clinical and Genetic Profile of Children With Neonatal Diabetes Mellitus
|
Case |
Age at |
Sex |
Clinical features |
Consan- |
Birth- |
Genetic analysis |
Diagnosis |
Treatment |
Follow-up |
|
No |
diagnosis |
|
|
guinity |
weight |
|
|
|
|
|
1 |
3 d |
F |
Hyperglycemia, Macroglossia, |
No |
2 kg |
Complete loss of methylation at |
TNDM |
Insulin × 5 mo, |
16 mo of age, |
|
|
|
umblicalhernia |
|
|
the TND differentially methylated |
|
then offinsulin |
off insulin, |
|
|
|
|
|
|
region on chromosome 6q24. |
|
|
doing well |
|
2 |
60 d |
M |
Polyuria, poor weightgain |
No |
2.4 kg |
Heterozygous missense mutation |
PNDM |
Insulin Initially, |
5 y, On |
|
|
|
|
|
|
(R201C) in the KCNJ11gene. |
|
Glibenclamide |
Glibenclamide, |
|
|
|
|
|
|
|
|
(0.5mg/kg) |
doing well |
|
3 |
160 d |
M |
Polyuria, seizures |
No |
2.5 kg |
Novel heterozygous frame- |
PNDM |
Insulin Initially, |
3 y, On |
|
|
|
|
|
|
deletionc.3808_3813delAACTCC |
|
Glibenclamide |
Glibenclamide, |
|
|
|
|
|
|
in exon 31 of the ABCC8 gene. |
|
(0.5mg/kg) |
doing well |
|
4 |
14 d |
F |
Polyuria, seizures |
2 degree |
3.6 kg |
Homozygous splicing mutation, |
PNDM |
Insulin Initially, |
3 y, on |
|
|
|
|
|
|
IVS16+1G>A, in intron 16 of the |
|
Glibenclamide |
Glibenclamide, |
|
|
|
|
|
|
ABCC8 gene; Father and mother carriers. |
|
(0.5mg/kg) |
doing well |
|
5 |
45 d |
M |
Polyuria, poor weightgain |
2 degree |
2.4kg |
Heterozygous missense mutation, |
PNDM |
Insulin |
3 y, On insulin, |
|
|
|
|
|
|
Y108D, inexon 3 of the INSgene. |
|
|
doing well |
|
6 |
90 d |
F |
Polyuria, FTT, DKA |
No |
2.3 kg |
Homozygous novel mutation. |
PNDM |
Insulin |
5 y, On insulin, |
|
|
|
|
|
|
c-218A>C/c.-218A>C, in the promoter |
|
|
doing well |
|
|
|
|
|
|
of the INS gene; Mother carrier. |
|
|
|
|
7 |
20 d |
F |
Poor feeding, lethargy, fever |
3 degree |
1.7 kg |
Homozygous for a novel missense |
PNDM |
Insulin |
8 y, on insulin, |
|
|
|
|
|
|
mutation, R176 Q in exon 2 of the |
|
|
doing well |
|
|
|
|
|
|
PDX1 (IPF1) gene. |
|
|
|
|
8 |
150 d |
M |
DKA (5 Months), |
2 degree |
3 kg |
Homozygous for a novel |
PNDM- |
Insulin, |
Died at 4 yrs |
|
|
|
Hepatitis (1,2 y), short |
|
|
missense mutation, R587Q, in |
Wolcott |
liver |
of age due to |
|
|
|
stature (2 y) |
|
|
exon 10 of the EIF2AK3 gene; |
Rallison |
supportive |
MODS |
|
|
|
|
|
|
Father and mother carriers. |
syndrome |
|
|
|
9 |
137 d |
F |
Polyuria, FTT, Anemia |
3 degree |
2.8 kg |
Heterozygous novel missense |
PNDM- |
Insulin, |
10 ys, on |
|
|
|
(8mo), Retinitispigmentosa |
|
|
mutation, G105 E in exon 2 of |
TRMA |
Thiamine |
insulin+thiamine, |
|
|
|
(7mo), cochlear implant (2 y) |
|
|
the SLC19A2 gene. |
|
|
Doing well |
|
10 |
60 d |
M |
DKA, Right focal seizure, |
2 degree |
2.4 kg |
Homozygous for a frame |
PNDM |
Insulin |
20 mo on |
|
|
|
inferior cerebellar vermis |
|
|
shift mutation c.235_236 insT, |
|
|
insulin, has |
|
|
|
hypoplasia |
|
|
in the NEUROD1gene. Father and |
|
|
mild motor |
|
|
|
|
|
|
mother carriers. |
|
|
developmental delay |
|
F- Female; M-Male; FTT- Failure to thrive; DKA- Diabetic
ketoacidosis; TNDM-Transient neonatal Diabetes mellitus;
PNDM-permanent Neonatal Diabetes mellitus. |
Discussion
Nine children were diagnosed with permanent NDM in
the present series. Heterozygous activating mutations in the KCNJ11
gene, that encodes the KATP channel subunit Kir6.2, accounts
for 47% of permanent NDM [5,6] and a few cases of treatment NDM
[7,8]. Similarly mutations in ABCC8 gene which encodes the SUR1
regulatory subunit of the ATP-sensitive potassium channels in beta cells
can cause both permanent and transient neonatal diabetes. In clinical
practice it is difficult to differentiate between patients with KCN
J11or ABCC8 mutations and oral sulfonyl urea becomes the
treatment of choice for diabetes resulting from both these mutations
[9,10]. Our patients were switched on treatment from insulin to oral
glibenclamide (0.5 mg/kg/day) once the genetic diagnosis was
established, and on follow up their glycemic control was good.
The present study describes the clinical and genetic
profile of children with neonatal diabetes mellitus. As the molecular
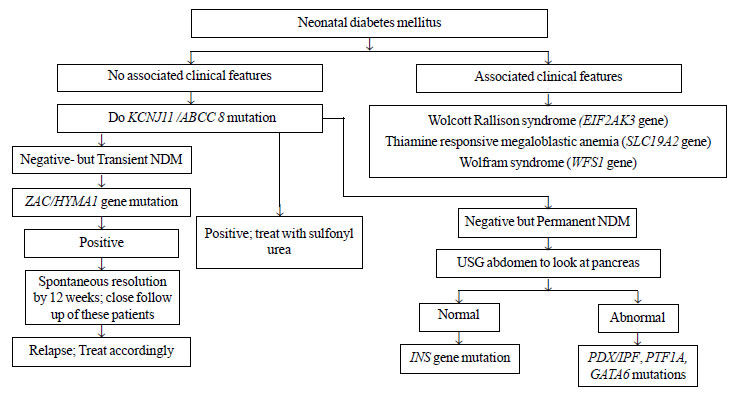
genetic testing is expensive, we suggest an algorithm to approach a
child with neonatal diabetes for ordering genetic testing in resource
limited setting like ours (Fig. 1). Molecular genetic
testing has a big impact on management of NDM as switching over to oral
sulfonyl urea is required in children with KCNJ11/ABCC 8 gene
mutation. Complete history, thorough clinical examination with a high
suspicion and correlation with physical findings may help us to guide
further the genotype work up of neonatal diabetes mellitus.
 |
|
Fig. 1 Proposed algorithm for
ordering genetic mutation testing in patients with neonatal
diabetes (NDM).
|
Acknowledgements: Prof Sian Ellard,
Consultant Molecular Geneticist, Peninsula Medical School (Royal Devon
and Exeter NHS Foundation Trust, Exeter) and Professor Karen Temple,
Wessex Regional Genetics laboratory, University of Southampton School of
Medicine for carrying out the molecular genetic work-up.
Contributors: RG, NS, TV: reviewed
literature, drafted manuscript and were involved in patient management;
KGR: reviewed manuscript for intellectual content and will act as the
guarantor.
Funding: None; Competing interests: None
stated.
|
What This Study Adds?
•
The present study reports the
molecular genetics of nine children with permanent neonatal
diabetes mellitus.
|
References
1. Hattersley A, Bruining J, Shield J, Njolstad P,
Donaghue KC. The diagnosis and management of monogenic diabetes in
children and adolescents. Pediatric Diabetes. 2009;10:33-42.
2. National diabetes information clearing house.
Monogenic Forms of Diabetes: Neonatal Diabetes Mellitus and
Maturity-onset Diabetes of the Young. Available from:
www.diabetes.niddk.nih.gov. Accessed February 24, 2012.
3. Slingerland AS. Monogenic diabetes in children and
young adults: Challenges for researcher, clinician and patient. Rev
Endocr Metab Disord. 2006;7:171-85.
4. Varadarajan P, Sangaralingam T, Senniappan S,
Jahnavi S, Radha V, Mohan V. Clinical profile and outcome of infantile
onset diabetes mellitus in southern India. Indian Pediatr.
2013;50:759-63.
5. Gloyn AL, Pearson ER, Antcliff JF, Proks P,
Bruining GJ, Slingerland AS, et al. Activating mutations in the
gene encoding the ATP-sensitive potassium channel subunit Kir6.2 and
permanent neonatal diabetes. N Engl J Med. 2004;350:1838-49.
6. Hattersley AT, Ashcroft FM. Activating mutations
in Kir6.2 and neonatal diabetes: new clinical syndromes, new scientific
insights, and new therapy. Diabetes. 2005; 54:2503-13.
7. Gloyn AL, Reimann F, Girard C, Edghill EL, Proks
P, Pearson ER, et al. Relapsing diabetes can result from
moderately activating mutations in KCNJ11. Hum Mol Genet.
2005;14:925-34.
8. Colombo C, Delvecchio M, Zecchino C, Faienza MF,
Cavallo L, Barbetti F. Transient neonatal diabetes mellitus is
associated with a recurrent (R201H) KCNJ11 (KIR6.2) mutation.
Diabetologia. 2005;48:2439-41.
9. Pearson ER, Flechtner I, Njolstad PR, Malecki MT,
Flanagan SE, Larkin B, et al. Switching from insulin to oral
sulfonylureas in patients with diabetes due to Kir6.2 mutations. N Engl
J Med. 2006;355:467-77.
10. Babenko AP, Polak M, Cave H, Busiah K, Czernichow
P, Scharfmann R, et al. Activating mutations in the ABCC8
gene in neonatal diabetes mellitus. N Engl J Med. 2006;355:456-66.
|
|
|
 |
|

