|
|
|
Indian Pediatr 2016;53:
70-72 |
 |
Gastrointestinal Neuroendocrine Tumors in Two
Children
|
|
Tugba Koca, Selim Dereci, *Nermin
Karahan and Mustafa Akcam
From Departments of Pediatrics, Division of Pediatric
Gastroenterology, Hepatology and Nutrition, and *Department of
Pathology; Faculty of Medicine, Suleyman Demirel University, Isparta,
Turkey.
Correspondence to: Dr Tugba Koca, Department of Pediatrics, Suleyman
Demirel University Faculty of Medicine, Cunur, Isparta, Turkey.
tgkoca@gmail.com
Received: February 23, 2015;
Initial review: April 27, 2015;
Accepted: October 27, 2015.
|
|
Background: Enterochromaffin-like cell hyperplasia and
neuroendocrine tumors are relatively rare in childhood.Case
characteristics: A 15-year-old girl who presented with epigastric
pain and a 6-year-old boy who was admitted with hematochezia. Endoscopy
revealed nodules in the stomach in Case 1, and polyploidy lesion in the
rectum in Case 2. Outcome: Enterochromaffin-like cell hyperplasia
in Case 1 and neuroendocrine tumor in Case 2. Message: A low
index of suspicion for neuroendocrine tumors in children can result in
delay in the detection of these rare but potentially malignant diseases.
Key words: Abdominal pain, H. pylori,
Hemtochezia.
|
|
Neuroendocrine tumors (NETs) – slow-growing tumors
that arise from cells within the neuroendocrine system — are rarely seen
in childhood [1,2]. The incidence in children and adolescents is low at
2.8 per million population under the age of 30 years. Despite their low
incidence, NETs represent the most frequent tumor of the
gastrointestinal tract in children [3].
Enterochromaffin-like (ECL) cell hyperplasia
constitutes the substrate for the development of gastric NETs type 1,
which represents the most common (65–75%) type of gastric NETs. There is
increasing diagnosis of NETs of the colon and rectum [4]. We present two
cases of NETs and ECL cell hyperplasia.
Case Reports
Case 1: A 15-year-old girl presented with a
3-month history of epigastric pain which was unresponsive to a 2-month
course of proton pump inhibitor (PPI). Physical examination was
unremarkable. Complete blood count, metabolic panel, lipase, C-reactive
protein, erythrocyte sedimentation rate, and celiac serology were
normal. Esophagogastroduodenoscopy revealed numerous 2 to 4 mm nodules
in the body of the stomach and in the transitional and pyloric region.
The histopathological investigation demonstrated focal intestinal
metaplasia and ECL cell hyperplasia in the stomach. Synaptophysin
staining highlighted an increase in ECL cells within the glands (linear
hyperplasia) and florets of ECL cells in the lamina propria (nodular
hyperplasia) (Fig. 1). Helicobacter pylori staining
was positive. Further laboratory evaluation revealed serum gastrin:
293.6 pmol/L (Normal 6.2-54.8), anti thyroglobulin: 322.7 IU/mL (Normal
0-115), and anti-Jo-1 positive. Serum vitamin B 123,
and free serum T4 levels
were normal. The PPI treatment was stopped after the result of the serum
gastrin level was available. An abdominal computed tomography scan was
normal. The secretin stimulation test was negative. Endoscopic and
pathological investigations were repeated one year after diagnosis.
H. pylori staining was negative and the histopathological findings
were similar to those seen on the earlier biopsies. In the second year
of follow-up, she had no significant abdominal pain. Third
esophagogastroduodenoscopy revealed multifocal atrophic gastritis and
focal intestinal metaplasia. ECL cell hyperplasia was identified in the
cardia, corpus and antrum-corpus junction. Additional laboratory reports
included anti-parietal cell antibodies positive and chromogranin A
elevated at 298.5 ng/mL (Normal 0-100). Serum gastrin level was high
(376 pmol/L) despite no use of PPI after the diagnosis. Given her
negative evaluation for H. pylori, positive anti-parietal cell
antibodies and gastric pathology, the patient was diagnosed with
autoimmune metaplastic atrophic gastritis (AMAG) with associated ECL
cell hyperplasia.
 |
|
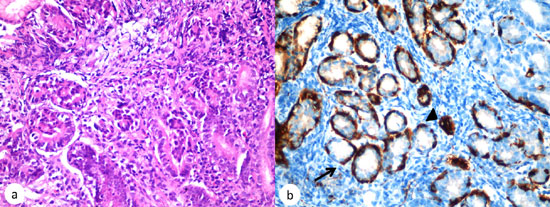
Fig. 1. (a)
Histopathology (Hematoxyline and eosin, X400) demonstrating
enterochromaffin-like (ECL) cell hyperplasia; (b),
Synaptophysin stain demonstrating both linear (arrow) and
nodular (arrowhead) patterns of ECL cell hyperplasia in the body
of the stomach (x 400).
|
Case 2: A 6-year-old boy was admitted with
abdominal pain for two weeks and hematochezia for 2 days. The physical
examination, upper gastrointestinal system endoscopy, routine laboratory
findings and Meckel scintigraphy were unremarkable. Colonoscopy revealed
polyploid architecture (0.6 cm in diameter) located in the rectum and 5
cm from the anal verge (Fig. 2a). Endoscopic resection was
performed and the histopathological examination was consistent with a
NET (Fig. 2 b and 2c). Hematochazia was not observed
during the hospitalization period. Due to the low risk of metastatic
spread, and as the tumors were small (<1 cm, without muscularis
invasion), we planned to perform endoscopic investigation every year.
 |
|
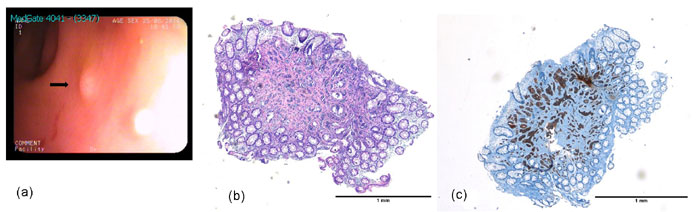
Fig. 2. (a) Endoscopic view of
polyploid architecture (0.6 cm in diameter) located in the
rectum (black arrow); (b) Hematoxylin and eosin stain of the
colon demonstrating NET; (c), Synaptophysin stain demonstrating
NET in the rectum.
|
Discussion
There are three situations in which clinicians
suspect ECL-cell hyperplasia. The simplest is when a nodule or polyp is
identified by the endoscopist. The second situation involves the
evaluation of gastric atrophy, and finally in PPI use as a result of
iatrogenic hypergastrinemia [5]. Initially, atrophic gastritis was not
determined in case 1, although she had nodules in the stomach with
history of PPI use.
Neuroendocrine nests are seen on the hematoxylin and
eosin-stained sections; there is no need to perform special staining.
ECL cells, typically found in the gastric corpus, are rare or absent in
other compartments of the stomach [5]. ECL cells were identified in the
cardia, corpus and antrum-corpus junction in Case 1. Long-standing
hypergastrinemia and, acid suppression exerts a proliferative pressure
on ECL cells, especially in the presence of H. pylori infection
[6]. The time of ECL hyperplasia regression after H. pylori
eradication is not known. In Case 1, there was PPI use together with
H. pylori infection. At follow-up, 1 year after diagnosis, H.
pylori was negative in the endoscopy examination but ECL had not
regressed. Surveillance endoscopic biopsy revealed H. pylori–negative
atrophic gastritis.
To date, there have been few published information
regarding ECL cell hyperplasia in children [7]. ECL hyperplasia together
with autoimmune thyroid disease and autoimmune gastritis has been
previously reported [8]. Anti-thyroglobulin antibody was positive in
case 1. Colonic and rectal NETs are also very rare in children [9]. Due
to the low risk of metastatic spread, tumors that are small and confined
to the mucosa or submucosa can be managed with endoscopic resection.
Hindgut NETs have a substantial risk of relapse after resection and need
to be followed up for at least 7 years [4]. In case 2, a small rectal
tumor (<1 cm, without muscularis invasion) was excised, and it was
planned to perform endoscopic investigation every year.
Chromogranin A levels correlate positively with ECL
cell mass in patients with AMAG [10]. Only a small fraction of hindgut
NETs produce and secrete serotonin or other bioactive hormones [3].
In conclusion, gastric NETs are usually solitary and
large and have frequently metastasized by the time of presentation.
Pediatric gastroenterologists and pathologists must be aware that NETs
can begin at a young age, and their immediate precursors such as ECL
cell hyperplasia develop in the pediatric age group.
Contributors: TK: management of cases; TK,
SD: wrote the manuscript; MC, NK: evaluated the histological
preparations; MAK: case management and final approval of manuscript.
Funding: None; Competing interest: None stated.
References
1. Li T, Qiu F, Qian ZR, Wan J, Qi XK, Wu BY.
Classification, clinicopathologic features and treatment of gastric
neuroendocrine tumors. World J Gastroenterol. 2014;20:118-25.
2. Sarvida ME, O’Dorisio MS. Neuroendocrine tumors in
children and young adults: Rare or not so rare. Endocrinol Metab Clin N
Am. 2011;40:65-80.
3. Howell DL, M. Sue O’Dorisio MS. Management of
neuroendocrine tumors in children, adolescents, and young adults. J
Pediatr Hematol Oncol. 2012;34:64-8.
4. Anthony LB, Strosberg JR, Klimstra DS, Maples WJ, O’Dorisio
TM, Warner RR, et al. The NANETS Consensus Guidelines for the
Diagnosis and Management of Gastrointestinal Neuroendocrine Tumors
(NETs): Well-differentiated nets of the distal colon and rectum.
Pancreas. 2010;39:767-74.
5. Cockburn AN, Morgan CJ, Genta RM. Neuroendocrine
Proliferations of the Stomach: A pragmatic approach for the perplexed
pathologist. Adv Anat Pathol. 2013;20:148-57.
6. Waldum HL, Hauso Ř, Fossmark R. The regulation of
gastric acid secretion - clinical perspectives. Acta Physiol (Oxf).
2014;210:239-56.
7. Yang R, Cheung MC, Zhuge Y, Armstrong C, Koniaris
LG, Sola JE. Primary solid tumors of the colon and rectum in the
pediatric patient: A review of 270 cases. J Surg Res. 2010;161:209-16.
8. Alexandraki KI, Nikolaou A, Thomas D, Syriou V, Korkolopoulou
P, Sougioultzis S, et al. Are patients with autoimmune thyroid
disease and autoimmune gastritis at risk of gastric neuroendocrine
neoplasms type 1? Clin Endocrinol (Oxf). 2014;80:685-90.
9. Johnson PR. Gastroenteropancreatic neuroendocrine
(carcinoid) tumors in children. Semin Pediatr Surg. 2014;23:91-5.
10. Peracchi M, Gebbia C, Basilisco G, Quatrini M,
Tarantino C, Vescarelli C, et al. Plasma chromogranin A in
patients with autoimmune chronic atrophic gastritis, enterochromaffin-like
cel lesions and gastric carcinoids. Eur J Endocrinol. 2005;152:443-8.
|
|
|
 |
|

