|
clinicopathological conference |
|
|
Indian Pediatr 2016;53: 51 -56 |
 |
An Adolescent with Kawasaki Disease
|
|
*Kirti Gupta, $Manojkumar
Rohit, #Avinash
Sharma, *Ritambhra Nada, ‡Sanjay
Jain and ^Subhash
Varma
Departments of *Histopathology, $Cardiology;
#Pediatric Allergy-immunology Unit, Department of Pediatrics, and
^Internal Medicine, Postgraduate Institute of Medical Education and
Research (PGIMER), Chandigarh. India.
Correspondence to: Dr Kirti Gupta, Additional
Professor, Department of Histopathology, Postgraduate Institute of
Medical Education and Research (PGIMER), Chandigarh, India.
Email:
kirtigupta10@yahoo.co.in
|
Kawasaki disease is an acute vasculitis of unknown
etiology that predominantly affects children <5 years of age. The
incidence and the severity of myocarditis in this disease is variable
and depends upon the stage of the disease, acute or chronic. Acute-stage
Kawasaki disease shows relatively high incidence of myocarditis, but
almost all cases are clinically mild. We describe teenage boy presenting
with atypical/incomplete manifestations of Kawasaki disease and
developing fulminant myocarditis within a week of illness resulting in
death. The case underscores the importance of suspecting Kawasaki
disease in a young child presenting with features of myocardial
ischemia.
Keywords: Complications, Incomplete Kawasaki disease,
Morbidity, Myocarditis.
|
|
Kawasaki disease is an acute febrile mucocutaneous
lymph node syndrome with multisystem vasculitis affecting infants and
children less than 5 years of age [1]. The diagnosis is established
clinically by the presence of six principal symptoms, including fever of
unknown etiology persisting for
³5
days, redness/desquamation of palms and soles, polymorphous exanthema,
conjunctival congestion, strawberry tongue and cervical lymphadeno-pathy
[2]. Children suspected of having Kawasaki disease with clinical or
laboratory features suggestive of the illness but who do not fulfil
diagnostic criteria (i.e., have less than four signs of mucocutaneous
inflammation) are said to have ‘incomplete’ or ‘atypical’ Kawasaki
disease [2-4]. The presentation in these patients is varied and not
always straightforward, leading to under-diagnosis or missed diagnosis.
As there is no available diagnostic test with sufficient specificity and
sensitivity for Kawasaki disease, in the absence of ‘classical’ clinical
symptoms, the diagnosis of Kawasaki disease requires a high index of
suspicion.
Clinical Protocol
History: A 14-year-old boy presented with
complaints of sudden onset chest pain and shortness of breath of one day
duration. The chest pain was left sided precordial, non-pleuritic
severe, squeezing and associated with sweating. It increased with
exertion and lying down but reduced with rest. This was associated with
shortness of breath (Class IV) and orthopnea. Shortness of breath was
followed by vomiting containing undigested food particles and an episode
of watery, loose stool. There was no history of fever, cough, syncope,
joint pain, sore throat, trauma, or drug intake. The past, family and
personal histories were non-contributory.
Clinical examination: He was afebrile, conscious,
but disoriented. He had a pulse rate of 120/min that was low volume. All
his peripheral pulses were palpable. His blood pressure was 90/60 mmHg
(right arm), respiratory rate of 26/min with SpO 2
91% on room air. There was no pallor, icterus, cyanosis, clubbing, or
adenopathy. He had pedal edema and raised jugular venous pressure (JVP).
There were subconjunctival hemorrhages in both eyes. Chest and
cardiovascular system examination was within normal limits. Liver and
spleen were not palpable, though ascites was present. Central nervous
system examination was normal. Troponin T was positive and creatine
kinase-MB was 197 unit on the day of admission; Lactate dehydrogenase
was elevated at 2215 unit on day 2. Serum cholesterol, Low-density
lipoprotein and triglycerides were normal. Blood and urine cultures were
sterile, Widal was negative; and peripheral smear for malaria parasite
was negative. Serology for Epstein-Barr Virus, Cytomegalovirus,
leptospira, Rickettsia and Dengue was negative. Antinuclear antibody
(ANA) was negative.
Radiology: Ultrasonography showed liver size of
15.1 cm, with normal echotexture, spleen size of 5.2 cm, and both
kidneys normal size and echotexture with moderate ascites.
Computed tomography pulmonary angiogram (CTPA) showed
a normal main pulmonary artery with all its branches. Inferior vena-cava
was dilated. Cardiac sections of CT showed ballooned out right atrium.
The left atrium was morphologically normal, the left ventricle wall
revealed concentric hypertrophy. Minimal pericardial effusion was noted
along the right atrium and right ventricle. Pulmonary artery was dilated
which was suggestive of pulmonary arterial hypertension (PAH). No
obstruction or mural thickening of the aorta was noted. Bilateral
pleural effusion was seen. No lymphadenopathy was noted.
Electrocardiogram (ECG) revealed following
findings on three consecutive days: Day 1 and 2- ST elevation in V 1,
q waves in lead I, aVL. No right ventricular hypertrophy (RVH)
identified. Pure R wave were noted in aVL. No R waves identified in V4
V5 and V6.
Day 3- Right bundle branch block (RBBB) was noted.
Echocardiography revealed aortic velocity 1.1,
Pulmonary velocity 1.0, and mild to moderate mitral regurgitation (MR)
(eccentric jet), moderate to severe tricuspid regurgitation (TR),
RVSP=RAP+34, and global hypokinesia. Estimated left ventricular ejection
fraction was 35-40%. There was mild pericardial effusion.
Course: The boy presented with acute onset
respiratory failure which was attributed to cardiac cause. He was
initiated on supportive care, which included sequential addition of
vasopressors and subsequent ventilation. He was started on intravenous
antimicrobials. He had one episode of generalized tonic-clonic seizure
during the stay. Injection heparin was started on day 2 of illness. He
started developing fever spikes on day 3 of admission. However, his
condition progressively deteriorated and he succumbed to his illness.
The postmortem blood culture grew Acinetobacter boumanii which
was sensitive to imipenem and colistin.
Unit’s Final Diagnosis: Viral myocarditis with
cardiogenic shock.
Discussion
Clinical discussant: We have a 14-year-old boy
who presented with complaints of acute chest pain and biventricular
failure. He initially had left ventricular failure (LVF) (marked
tachycardia, marked tachypnea, hypotension and hypoxemia) and later
developed right ventricular failure (RVF) (hepatomegaly, raised JVP,
dilated RA and RV, and ascites). ECG showed lateral wall changes along
with elevated CPK-MB, increased troponin-T and increased LDH. During his
hospital stay, he had mild elevation of AST which increased
significantly likely due to systemic hypotension. He had one episode of
seizure which was most likely secondary to hypoxia, and pre-terminally
developed febrile illness with worsening hemodynamics.
TABLE I Results of Hematological and Biochemical Investigations in the Index Case
|
Investigations |
Day 1 |
Day 2 |
Day 3 |
|
Hemoglobin (g/dL) |
13.9 |
|
10.4 |
|
Total leukocyte count (109/L) |
10.1 |
|
13.2 |
|
Platelets (109/L) |
393 |
|
220 |
|
Serum sodium (meq/L) |
138 |
140 |
141 |
|
Serum potassium (meq/L) |
4.9 |
3.8 |
4.2 |
|
Blood urea (mg/dL) |
37 |
60.7 |
62.7 |
|
Serum creatinine (mg/dL) |
0.67 |
0.84 |
0.73 |
|
*AST (IU/L) |
360 |
1441 |
1507 |
|
#ALT (IU/L) |
286 |
1241 |
1572 |
|
Alkaline phosphatase (U/L) |
271 |
256 |
195 |
|
Serum bilirubin total (mg/dL) |
1.85 |
2.9 |
2.7 |
|
Serum bilirubin conjugated (mg/dL) |
0.31 |
0.6 |
0.53 |
|
Serum calcium (mg/dL) |
10.1 |
8.01 |
|
|
Serum phosphorus (mg/dL) |
5.4 |
3.44 |
|
|
Serum total proteins (g/dL) |
5.84 |
6.43 |
|
|
Serum albumin (g/dL) |
3.9 |
4.5 |
|
|
*Aspartate aminotransferase; #Alaline aminotransferase. |
Acute onset chest pain with biventricular failure,
ECG evidence of myocardial ischemia, elevated CPK-MB, and increased
toponin-T point to myocardial ischemia as the predominant
pathophysiology in this child. Myocardial ischemia in children and
adolescents is rare and can occur due to the following causes:
• Aortic stenosis: Absence of aortic stenosis on
ECHO and LVH on ECG go against this diagnosis.
• Congenital coronary artery abnormalities
Anomalous left coronary artery from the pulmonary
artery (ALCAPA): ALCAPA usually presents in infancy, but 10-15% patients
will present late with myocardial dysfunction and with ECG showing
typical features of Q waves in lead I, T wave inversion in lead I, aVL
and poor R wave in V 4, V5
and V6. This is an important
cause of left sided myocardial ischemia in children and should be
thought of any child who presents with ischemic changes in left sided
leads.
Anomalous origin of left coronary artery from right
coronary sinus: This left coronary artery after origin from right
coronary sinus courses between aorta and pulmonary artery which can
cause ischemia. Clinical presentation is generally with arrhythmia and
acute LVF is usually not seen. Still it should be considered in any
young person with chest pain as a remote possibility.
Other coronary artery abnormalities like coronary
artery fistula are very rare and are difficult to diagnose clinically.
Coronary fistula is usually asymptomatic at this age. These patients do
not present acutely unless a catastrophe like thrombotic occlusion
occurs. An easily audible continuous murmur helps in the diagnosis.
• Acquired causes of coronary artery involvement:
Among the acquired causes of myocardial infarction, Kawasaki
disease, coronary arteritis and coronary artery dissection are known
to occur in young children. Drug abuse also remains a possibility.
Kawasaki disease is the most important cause of
myocardial ischemia in children. A past history of Kawasaki disease can
easily be mistaken as a viral illness. Upto 25% of untreated children
with Kawasaki disease will develop coronary artery abnormalities. Giant
coronary aneurysms with complications like thrombosis, stenosis and
rupture are well known. So, this possibility should always be considered
in such a child. ECHO can pick up coronary abnormalities and help in
diagnosis.
Coronary arteritis has been described with many
systemic illnesses like Systemic lupus erythematosus (SLE) and
Polyarteritis nodosa (PAN). This is a possibility; however, there are no
clinical features to suspect these conditions. Spontaneous dissection of
coronary artery is more common in young pregnant females. There have
been case reports in young children who present with sudden onset of
symptoms. Drug abuse causing coronary vasospasm and resultant MI remains
a possibility, though there is no such history available.
Besides coronary abnormalities, myocardial ischemia
can be caused by myocardial diseases; hypertrophic cardiomyopathy being
the commonest. Absence of septal hypertrophy on echocardiography and LVH
on ECG go against this diagnosis.
Myocarditis should also be considered in such a
setting, as it can masquerade acute coronary events. Elevation of muscle
enzymes is well known and diffuse ECG changes like ST elevation in lead
I, aVL, and ST depression in lead III, aVF, and poor R waves are well
described in myocarditis. Echocardiography finding of global hypokinesia
is also seen in myocarditis. With a short history, elevated enzymes and
ECG changes, it remains a strong possibility. Fulminant myocarditis can
have a similar presentation and important causes include infections,
toxins, hypersensitivity myocarditis, immune mediated disorders,
necrotizing eosinophilic myocarditis and giant cell myocarditis.
Clinically, it is practically impossible to pinpoint a specific cause of
myocarditis.
Besides left ventricular failure, this child also had
ascites and edema along with right atrial and right ventricular
dilatation, severe tricuspid requrgitation and no pulmonary embolism. It
is difficult to explain prominent right ventricular failure based on
myocarditis or myocardial ischemia. So the question is whether this
child had an underlying right ventricular dysfunction which was
asymptomatic till date and manifested with myocardial ischemia/myocarditis.
Among the myocardial diseases responsible for right
ventricular dysfunction, arrhythmogenic right ventricular dysplasia
commonly presents with arrhythmia. There were no clinical features to
suggest Fabry’s disease. Uhl’s anomaly is a rare disorder that can
present in infancy with RVF. It seems likely that this child did not
have a myocardial cause for his RVF. Right ventricular ischemia remains
an important cause for RVF in adults. Index child did not have ECG
evidence of RV infarction. ECHO did not show evidence of tricuspid
regurgitation or stenosis either. It is possible to miss sinus venosus
type of atrial septal defect (SVC ASD) on routine echocardiography.
Sinus of Valsalva aneurysms can present at this age with bi-ventricular
failure, but this entity is marked by a continuous murmur and this child
did not have one.
Pressure overload situations like severe pulmonary
artery hypertension and pulmonary embolism have been ruled out by CT
scan. Right ventricular outflow tract obstruction (RVOT) has been ruled
out by echocardiography. So, pulmonary vascular diseases seem unlikely.
He did not have any features of RVH. Pericardial diseases can present
with biventricular failure, however, it seems unlikely in this child.
Pre-terminally, he had healthcare-associated
infection with persistent fever and positive blood culture.
So, my final diagnosis is acute fulminant myocarditis,
likely viral or myocardial infarction with premature coronary artery
disease like dissection or giant coronary artery aneurysm and cause of
death is healthcare-associated infection.
Clinician in-charge: This child presented a
diagnostic challenge of acute onset bi-ventricular failure with
predominant RVF features. Most likely it was an acute on chronic event.
Looking at the fulminant presentation, viral myocarditis was the first
possibility based on which intravenous immune globulin (IVIg) was given
to this child. It was not clear whether there was any underlying
congenital or acquired myocardial disease.
Chairman: This child played football the previous
evening, meaning thereby that even if he had an underlying heart
disease, it was fairly well compensated and an acute event caused
decompensation.
Physician 1: This child had leucocytosis, edema,
ascites, sub-conjunctival hemorrhage, transaminitis and anemia. A
possibility of leptospirosis could be considered but the odd points are
the absence of fever and absence of renal involvement.
Chairman: The absence of fever is a very
important finding. As we know, leucocytosis is an acute phase response
which can occur in multiple conditions including non-infectious like
myocardial ischemia.
Physician 2: In view of the global involvement of
myocardium as demonstrated by echocardiogram, I would consider
myocarditis as the possibility rather than myocardial ischemia which
will cause a focal involvement.
Cardiologist: There is discordance between the
history, clinical examination and the investigation. With this acute
illness, ECG and echocardography showing global involvement, myocarditis
remains the first possibility but leptospirosis has to be looked for.
Pediatrician 1: A young boy coming with
myocarditis or MI like presentation, Kawasaki disease remains as one of
the most important possibility. Atypical and incomplete presentations
are well described in the literature.
Radiologist: With myocardial thickening and RA,
RV dilatation, possibility of myocarditis is strong.
Pathology protocol
A partial autopsy was carried out. Externally, the
deceased was noted to be thin-built. There was pericardial effusion and
pleural effusion with 50 mL and 500 mL of straw-colored fluid,
respectively. The peritoneal cavity was within normal limits. The
findings in the heart were remarkable with biventricular dilatation and
bifid apex. Thrombus was noted in the right auricle. All the chambers
were dilated. The right and left ventricular wall thickness measured 0.6
cm and 1.6 cm, respectively. On gross examination, there was transmural
haemorrhagic discoloration of ventricular wall involving papillary
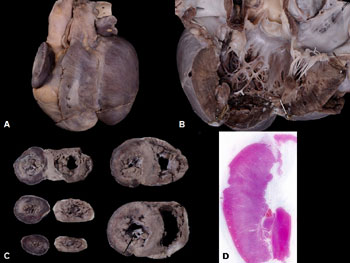
muscle and major portions of RV (Fig. 1). All the four
valves were within normal limits. On microscopy, there was fibrinous
pericarditis, extensive interstitial myocarditis with a neutrophil-rich
infiltrate and perivasculitis involving major portions of both
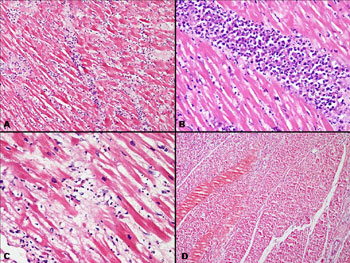
ventricles (Fig. 2). Features of myocardial ischemia with
loss of nuclei, hyper-eosinophilia and significant inter-myocyte edema
were detected in major portions of left and in parts of right ventricle
(Fig. 2d). Coronaries and other medium-sized vessels (Web
Fig. 1) (superior mesenteric and right external iliac artery)
demonstrated extensive disruption of internal elastic lamina (IEL) with
irregular heaping of intima. Fibrinoid necrosis and significant medial
inflammation were not detected within the vessel walls. No aneurysms
were identified. The polymerase chain reaction (PCR) for Coxscakie and
entero-viruses performed on the DNA extracted from the post-mortem heart
and kidney tissue was negative. Lungs revealed features of early
bronchopneumonia with focal alveolar hemorrhages. The rest of the organs
examined both grossly and microscopically were within normal limits.
 |
|
Fig. 1 (a) Gross appearance of heart
from anterior surface with bifid apex. (b) Dilated left
ventricle with transmural haemorrhagic discoloration of
ventricular wall and endocardial sclerosis. (c) Serial
horizontal slices of heart from apex to base with transmural
haemorrhagic discoloration involving both ventricles and
portions of interventricular septum. (d) Scanner view of left
ventricle (LV) demonstrates large areas of coagulative necrosis
of its myocardium and papillary muscle.
|
| |
 |
|
Fig.2 (a) Interstitial myocarditis
with a neutrophil predominant infiltrate and interstitial oedema
(H&E×100), (b) Dense interstitial collection of inflammatory
cells (H&E×200), (c) Inflammatory cells with myocyte destruction
(H&E×400), (d) Large areas of infracted myocardium with loss of
myocyte nuclei, loss of striations and hypereosinophilia of
fibres (H&E ×100).
|
Final Autopsy Diagnosis: Kawasaki disease with
early bronchopneumonia and pleural effusion
Open Forum
Pediatrician 1: Myocarditis is a universal
feature in Kawasaki disease as documented in many previous autopsy
series. So, myocarditis accompanied by vasculitis and infarction, I
think Kawasaki disease remains a strong possibility.
Pathology discussant: The findings which favoured
a diagnosis of Kawasaki disease were myocardial ischemia and involvement
of coronaries, i.e. the destruction of the internal elastic lamina which
is a key feature and an early event for the aneurysm formation. The
history lasted only three days and had he lived long, we could have
found other features as well.
Chairman: In a young child or adolescent
presenting with features of heart failure and ischemia, possibility of
Kawasaki disease should be considered more frequently.
Discussion
Kawasaki disease is an important childhood systemic
vasculitis with potentially major cardiovascular implications,
especially if the diagnosis is missed, and if timely and appropriate
treatment is not given. The incidence of incomplete Kawasaki disease is
unknown [5,6]. In a retrospective report of 242 Japanese children with
Kawasaki disease treated at a single center over a nine-year period, 10
percent of patients were diagnosed with incomplete Kawasaki disease [5].
The incidence appears to be greater in infants younger than six months
of age [6,7].
Pathological studies over the years on Kawasaki
disease have mostly focused on arterial lesions with changes mostly
affecting the coronary arteries [8-10]. Ischemic cardiac lesions due to
stenosis, thrombosis or aneurysmal dilatation of coronary artery have
been well described within the spectrum of acute or chronic-phase
Kawasaki disease [10]. Myocarditis frequently occurs in the acute phase
of disease; however almost all cases are clinically mild [2,11].
Fulminant fatal myocarditis during the acute-phase of the disease is
rarely reported in the literature. While the clinical presentation in
the teenager boy was atypical or incomplete for Kawasaki disease, the
presence of subconjunctival hemorrhages was the positive clinical clue
which was unfortunately missed. Histologically, presence of acute
interstitial myocarditis accompanied by coronary arteritis is considered
to be characteristic myocardial lesion in Kawasaki disease [12].
Myocarditis in Kawasaki disease have been described to develop prior to
development of epicardial coronary arteritis as early as on the sixth
day of onset of illness in a recent study on autopsied hearts [12]. The
inflammatory infiltrate in Kawasaki disease chiefly comprises of
neutrophils and monocytes/macrophages and few lymphocytes, in contrast
to viral myocarditis wherein the infiltrate is mostly composed of
lymphocytes accompanied by necrosis of individual myocardial cells or
myocardial fibre bundles [13].
Coronary arteritis as seen in this case is
characterized by irregular thickening of the initima along with breakage
of the internal elastic lamina, which is the key feature for aneurysm
formation noted in 20-25% of cases [14]. The rapidly fulminant fatal
course perhaps preluded the development of aneurysms in the teenager
boy. The differential diagnoses include common childhood febrile
illnesses like measles, scarlet fever and viral fever. Coronary artery
abnormalities (CAA) that occur in Kawasaki disease can lead to long term
consequences in the form of thrombosis, stenosis or occlusion leading to
MI, ischemia or sudden death. There have been multiple reports and
studies reporting patients who had coronary events as long term sequelae
attributable to childhood Kawasaki disease [15-18].
The case underscores the importance of suspecting
Kawasaki disease in a young child presenting with features of myocardial
ischemia.
References
1. Kawasaki T, Kosaki F, Okawa S, Shigematsu I,
Yanagawa H. A new infantile acute febrile mucocutaneous lymph node
syndrome (MLNS) prevailing in Japan. Pediatrics. 1974, 54: 271-6.
2. Newburger JW, Takahashi M, Gerber MA, Gewitz MH,
Tani LY, Burns JC, et al. Diagnosis, treatment, and long-term
management of Kawasaki disease: A statement for health professionals
from the Committee on Rheumatic Fever, Endocarditis and Kawasaki
Disease, Council on Cardiovascular Disease in the Young, American Heart
Association. Pediatrics. 2004; 114:1708-33.
3. Burns JC, Glodé MP. Kawasaki syndrome. Lancet.
2004;364:533-44.
4. Cimaz R, Sundel R. Atypical and incomplete
Kawasaki disease. Best Pract Res Clin Rheumatol. 2009;23:689-97.
5. Fukushige J, Takahashi N, Ueda Y, Ueda K.
Incidence and clinical features of incomplete Kawasaki disease. Acta
Paediatr. 1994;83:1057-60.
6. Joffe A, Kabani A, Jadavji T. Atypical and
complicated Kawasaki disease in infants. Do we need criteria? West J
Med. 1995,162:322-7.
7. Rosenfeld EA, Corydon KE, Shulman ST. Kawasaki
disease in infants less than one year of age. J Pediatr. 1995;126:524-9.
8. Naoe S, Takahashi K, Masuda H, Tanaka N. Kawasaki
disease with particular emphasis on arterial lesions. Acta Pathol Jpn.
1991;41:785-97.
9. Masuda H, Naoe S, Tanaka N. A pathological study
of coronary artery in Kawasaki disease (MCLS) with special reference to
morphogenesis. J Jpn Coll Angiol. 1981;21:899-912.
10. Fujiwara H, Hamashima Y. Pathology of the heart
in Kawasaki disease. Pediatrics. 1978;61:100–7.
11. Takahashi M. Myocarditis in Kawasaki syndrome.
Circulation. 1989;79:1398-400.
12. Harada M, Yokouchi Y, Oharaseki T, Matsui K,
Tobayama H, Tanaka N, et al. Histopathological characteristics of
myocarditis in acute-phase Kawasaki disease. Histopathology.
2012;61:1156-67.
13. Kindermann I, Barth C, Mahfoud F, Ukena C, Lenski
M, Yilmaz A, et al. Update on myocarditis. J Am Coll Cardiol.
2012;59:779-92.
14. Kato H, Inoue O, Kawasaki T , Fujiwara H,
Watanabe T, Toshima H. Adult coronary artery disease probably due to
childhood Kawasaki disease. Lancet. 1992;340:1127–9.
15. Suda K, Tahara N, Kudo Y, Yoshimoto H, Iemura M,
Ueno T, et al. Persistent coronary arterial inflammation in a
patient long after the onset of Kawasaki disease. Int J Cardiol.
2012;154:193-4.
16. Daniels LB, Tjajadi MS, Walford HH,
Jimenez-Fernandez S, Trofimenko V, Fick DB Jr, et al. Prevalence
of Kawasaki disease in young adults with suspected myocardial ischemia.
Circulation. 2012;125:2447-53.
17. Tsuda E, Hirata T, Matsuo O, Abe T, Sugiyama H,
Yamada O, et al. The 30-year outcome for patients after
myocardial infarction due to coronary artery lesions caused by Kawasaki
disease. Pediatr Cardiol. 2011;32:176-82.
18. Tsuda E, Abe T, Tamaki W. Acute coronary syndrome
in adult patients with coronary artery lesions caused by Kawasaki
disease: review of case reports. Cardiol Young. 2011;21:74-82.
|
|
|
 |
|

