|
|
|
Indian Pediatr 2015;53: 15-17 |
 |
Centralized Newborn Hearing Screening in
Ernakulam, Kerala – Experience Over a Decade
|
|
Abraham K Paul
Convenor, Newborn Hearing Screening Programme-IAP,
Kochi, Kerala.
Correspondence to: Dr Abraham K Paul, Pediatrician,
Cochin Hospital, Cochin-16, Kerala.
Email: abrahamkpaul@gmail.com
|
|
A two-stage centralized newborn
screening program was initiated in Cochin in January 2003. Infants are
screened first with otoacoustic emission (OAE). Infants who fail OAE on
two occasions are screened with auditory brainstem response (ABR). All
Neonatal intensive care unit babies undergo ABR. This successful model
subsequently got expanded to the whole district of Ernakulam, and some
hospitals in Kottayam and Thrissur districts. Over the past 11 years,
1,01,688 babies were screened. Permanent hearing loss was confirmed in
162 infants (1.6 per 1000). This practical model of centralized newborn
hearing screening may be replicated in other districts of our country or
in other developing countries.
Keywords: Disability, Hearing loss, Neonate,
Prevention, Universal newborn hearing screening.
|
|
Hearing loss is one of the most common anomalies,
occurring in 1-2 per 1000 infants [1]. The incidence is considerably
higher in infants in the Neonatal intensive care unit (NICU) (1-2 cases
per 200 infants) [2]. Left undetected, hearing impairment in infants can
negatively affect speech and language acquisition, academic achievement
and social and emotional development. These negative effects can be
diminished and even eliminated through early intervention at or before 6
months of age [3]. Neonatal hearing loss and its developmental
consequences are measurable before the age of 3 years [4-6]. It is an
established fact that language development is positively and
significantly affected by age of identification of hearing loss and age
of initiation into intervention services.
Reliable screening tests that minimize referral rates
and maximize sensitivity and specificity are available. The goal of
Universal neonatal hearing screening is to maximize linguistic and
communicative competence and literacy development for children who are
hard of hearing or deaf.
American Academy of Pediatrics (AAP) in 1999
advocated Universal new born hearing screening programme and remedial
intervention which is being practiced in most of the developed
countries. In a developing country like India, the risk of infants to
develop these difficulties is obviously more [8,9]. In interventional
programs, the Indian studies mostly cite the screening facilities
available to newborns brought in to tertiary referral hospitals [10-12].
A hearing screening equipment facility in every hospital with maternity
units today may not be a viable proposition. In this background, a
practical interventional model was conceived for the city of Cochin
(which has 20 hospitals with maternity units) in January 2003.
Centralized Newborn Hearing Screening
A two-stage screening protocol with Otoacoustic
emission (OAE) as the first screen, followed by Auditory brainstem
response (ABR) for those who fail the second OAE screen was introduced.
All NICU babies underwent ABR. With three portable screening machines
and three screeners, 20 hospitals in the city of Cochin became partners
in the program. This practical interventional model of centralized
newborn screening was found to be a cost-effective solution to the
newborn hearing screening of Cochin city [13]. After a decade of
successful operation, it was decided to expand the program to whole of
Ernakulam District with 91 hospitals, and part of hospitals in
neighboring Kottayam and Thrissur districts. Thirteen hospitals had
their own inhouse screening facility. Five more machines were procured
and another five personnel trained and appointed. The program became
operational in August 2014. The programme is co-ordinated by a speech
and language pathologist and weekly assessment meeting is convened with
the staff by the convenor.
Screening facility operates out of Child Care Centre,
which is also the secretariat of IAP Cochin Branch. Personnel with basic
knowledge in computer and good communication skills were chosen, given
basic training in hearing screening and also skill to gather information
of high risk criteria, if any, from parents/hospital staff/ hospital
records. The screening personnel visit each hospital daily/alternate
days/twice-a-week/weekly depending upon the number of births in that
particular hospital. Daily screening was carried out in hospitals which
had more than 200 births, alternate day screening in hospitals with
100-200 births and twice weekly or weekly screening in hospitals with
births less than 100 per month. On an average, each staff screen about
10-20 babies per day depending upon the hospital delivery rate. If
abnormal OAE, it is repeated at 6 weeks on the 1 st
immunization visit. If again abnormal, ABR is done for confirmation
followed by full audiological evaluation and remediation. All NICU
babies undergo ABR testing. In babies with abnormal ABR, detailed
enquiry is made to identify and record any risk factors [14]. Any baby
missing screening before hospital discharge is called for OAE test on
the first immunization visit [13]. The salary for staff, cost of
equipments and consumables are met from the testing fees (Rs.150 per
baby) collected.
There were a total of number of 1,20,630 births in 78
hospitals over the period from January 2003 to January 2015, out of
which 82,268 babies underwent screening before discharge and 19,420
babies at 6 weeks. The number of births in individual hospitals varied
from 15 per month to 275 per month. Two hospitals had births more than
200, 9 had between 100 – 200 and 67 hospitals below 100. A total of
18,942 babies missed screening and the majority were from hospitals
where screening was done on a weekly basis.
In 13 major hospitals that had in-house OAE screening
facility, the screening was done by audiologists and they had their own
ABR facility. Out of 78 hospitals where screening was done by our team,
11 had ABR facility and the others were depending on nearby private
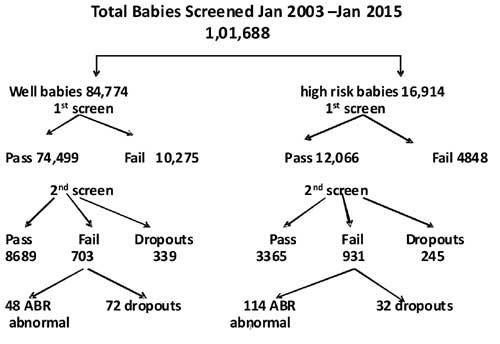
hospitals or ENT/Audiology centers for the same. Out of 1,01,688 babies
screened, 16,914 were in the high-risk group and 84,774 were not in
high-risk group. Out of 84,774 babies in the non-high risk group, 339
were dropouts for second OAE Screen and 72 for ABR screening. They
failed to return even after repeated phone calls. In the high-risk
group, 245 were dropouts for second OAE screen and 32 were dropouts for
ABR screening (Fig. 1). The mean interval between
OAE fail and ABR testing was four weeks.
 |
|
Fig. 1 Result of newborn hearing
screening for high-risk and well babies.
|
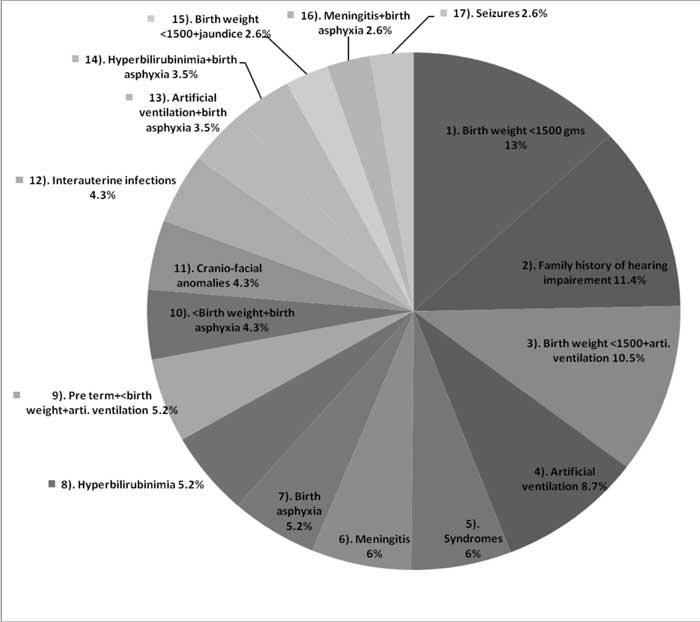
48 infants (29.6%) had no risk factors and 114 babies
had one or more risk factors. Risk factors for hearing loss were
identified by using the guidelines of the Joint Committee on Infant
Hearing 2000 position statement [14]. The distribution of different risk
factors are presented in Fig. 2.
 |
|
Fig. 2. Distribution of risk factors
according to guidelines of Joint Committee on Infant Hearing
2000 position statement.
|
The most common risk-factor was low birth weight
(13%) followed by familial deafness (11%). Low birth weight coupled with
mechanical ventilation contributed to 10.5% and mechanical ventilation
alone accounted for 8.8%. Hyperbilirubinimia alone contributed to 5.3%
cases.
Ernakulam District Experience
Out of 1,01,688 cases screened over the past 11
years, 84,774 were the well-baby nursery group. Out of that, 10,275
failed the first OAE screen. This 12% failure may be an acceptable
figure because of early screening on Day 2 or 3 of delivery in view of
the early discharge practice. 72 dropouts for ABR out of 703 who failed
the second OAE screen is a matter of concern. A matter of more concern
is the drop-outs in the high risk group even after repeated reminders
(245 out of 4848 who failed the first OAE screen and the 32 dropouts
after the second OAE screen). The incidence of congenital deafness in
well baby nursery in our study is 0.6 per thousand as compared to 1 in
1000 in the literature [1]. In the high-risk population of 16,914 babies
screened, 114 babies were detected to have congenital hearing loss with
an incidence of 0.7 per 100 as compared to 1-2 cases per 200 [2].
The most common cause of congenital deafness in our
series was birth weight <1500 gms (13%) followed by familial deafness in
11%. This is in contrast to observation by Declau, et al. [7]
screening 87,000 newborns in a tertiary care center in Belgium, where
the most common etiological factor was familial deafness accounting for
10.6%. 35 out of 114 cases of permanent congenital hearing loss had more
than 1 risk factors (30%).
Conclusion
Universal Newborn Hearing Screening (UNHS) has become
a standard practice in most developed countries. The identification of
all newborns with hearing loss before six months has now become an
attainable and realistic goal, as our program of universal newborn
hearing screening in Ernakulam District crosses one lakh babies. The
concept of a centralized new born hearing screening model to cater to
all hospitals in the districts is worth replicating. It takes away the
financial burden of each hospital investing for the screening equipment.
Follow up of positive cases and drop-outs is made easier with the
central reporting and monitoring system. With unified strength of
pediatricians, IAP city/district branches could take initiative to
replicate this model in their respective towns or districts.
References
1. Parving A, Hauch AM, Christensen B. Hearing loss
in children: epidemiology, age at identification and causes through 30
years [in Danish]. Ugeskr Laeger. 2003;165:574-9.
2. Van Straaten HL, Tibosch CH, Dorrepaal C, Dekker
FW, Kok JH. Efficacy of automated auditory brainstem response hearing
screening in very preterm newborns. J Pediatr. 2001;138:674-8.
3. Yoshinaga-Itano C, Coulter D, Thomson V.
Developmental outcomes of children with hearing loss born in Colorado
hospitals with and without universal newborn hearing screening programs.
Semin Neonatol. 2001;6:521 -9.
4. Yoshinaga-Itano C, Apuzzo ML. Identification of
hearing loss after age 18 months is not early enough. Am Ann Deaf.
1998;143:380-7.
5. Yoshinaga-Itano C, Apuzzo ML. The development of
deaf and hard of hearing children identified early enough through the
high risk registry. Am Ann Deaf. 1998;143:416-24.
6. Fortnum HM, Summerfieled AQ, Marshal DH, Davis A,
Bamford M. Prevalence of permanent childhood hearing impairment in
United Kingdom and implications for universal neonatal hearing
screening: Questioner based assertainment study. BMJ. 2001;323:536-40.
7. Declau F, AA Boudewayns, Jenneke Van deu Ende,
Peters A. Etiologic and audiologic evaluations after universal neonatal
hearing screening: analysis of 170 referred neonates. Pediatrics.
2008;121:1119-1126.
8. Report of the Collective Study on Prevalence and
Etiology of Hearing Impairment. New Delhi: ICMR and Department of
Science; 1983.
9. Kacker SK. The Scope of Pediatric Audiology in
India. In: Deka RC, Kacker SK, Vijayalakshmi B, eds. Pediatric
Audiology in India, 1st ed. New Delhi: Otorhinolaryngo-logical Research
Society of AIMS; 1997.p.20.
10. Nagapoonima P, Ramesh A, Srilakshmi, Rao S,
Patricia PL, Gore M. Universal hearing screening. Indian J Pediatr.
2007;74:545-8.
11. Vaid N, Shanbag J, Nikam R, Biswas A. Neonatal
hearing screening – The Indian experience. Cochlear Implants Int.
2009;10:111-4.
12. Ramesh A, Nagapoornima M, Srilakshmi V, Dominic
M. Swarnarekha. Guidelines to Establish a Hospital-based Neonatal
Hearing Screening Programme in the Indian Setting. JAIISH.
2008;27:105-9.
13. Paul AK. Early identification of hearing loss and
centralized newborn hearing screening facility-The Cochin experience.
Indian Pediatr. 2011;48:356-9.
14. Joint Committee on Infant Hearing; American
Academy of Audiology, American Academy of Pediatrics; American
Speech-Language-Hearing Association; Directors of Speech and Hearing
Programmes in State Health and Welfare Agencies. Year 2000 Position
Statement: Principles and Guidelines for Early Hearing Detection and
Intervention Programmes. Pediatrics. 2000;106:798-817.
|
|
|
 |
|

