|
|
|
Indian Pediatr 2015;52: 78 -79 |
 |
Albendazole-induced Autoimmune Hepatitis
|
|
*Tugba Koca and Mustafa Akcam
Department of Pediatrics, Süleyman Demirel University
School of Medicine, Cunur, Isparta, Turkey.
Email: [email protected]
|
|
There are no published data on drug-induced autoimmune hepatitis caused
by albendazole. We present here a patient with autoimmune hepatitis
(AIH) induced by albendazole prescribed for hydatid cyst. A six-year-old
girl was referred to our outpatient clinic with the diagnosis of liver
hydatid cyst. Her physical examination and routine laboratory analyses
were unremarkable. Albendazole treatment (15 mg/kg/day) was given for
two weeks to perform puncture, aspiration, injection and re-aspiration
(PAIR). But she was lost to follow up and she was admitted with
abdominal pain after 2 months. AST, ALT, and GGT were 663, 800, and 92
IU/L, respectively. Laboratory investigations to exclude infectious,
autoimmune, and metabolic liver disease were normal. The elevated
transaminase levels returned to the normal range after cessation of
albendazole. At 9
th month,
abdominal ultrasound revealed a progressive increase in the size of
the cyst. Treatment with PAIR technique was considered. Albendazole
treatment (15 mg/kg/day) was initiated again 2 weeks before the PAIR
procedure. She had mild elevation of transaminase levels at time of the
procedure, but albendazole was continued for a week. Three weeks later,
AST and ALT were 496 and 468 IU/L. ANA titers became positive (1:100;
granular pattern). The pattern of liver enzyme derangement in the child
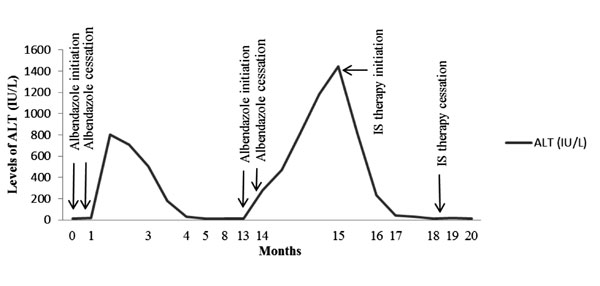
is depicted in Fig. 1. Liver biopsy showed widespread
portal lymphoplasmacytic inflammation with extensive interface
(piecemeal) necrosis. Prednisolone and azathioprine were started.
Transaminase levels rapidly decreased to normal ranges in two weeks. One
month later, she had no complaints; physical examination and laboratory
parameters were all normal. The steroid dose was tapered. Four months
later, laboratory findings and clinical features were also normal.
Afterwards, the dose of azathioprine was also tapered.
 |
|
Fig. 1 The pattern of liver enzyme
derangement in the patient.
|
AIH can be triggered in susceptible persons by an
external factor. Previous data suggest [2] that drug-induced AIH makes
up a significant proportion, approximately 9%, of AIH cases [1].
Björnsson, et al. [2] suggested that a substantial number of
patients who were found to develop drug-induced liver injury were
diagnosed with AIH during follow-up.
Our patient had transaminitis recurring every time
after treatment of albendazole. In the first episode, elevated
transaminase levels rapidly returned to the normal ranges following the
cessation of albendazole. Also, ANA was negative and IgG level was in
normal range. Hence, she was diagnosed as drug-induced hepatotoxicity
due to albendazole. AIH was considered during second episode as ANA
became positive, IgG level raised, and liver biopsy showed histologic
features of AIH. Rapid response to immunosuppressive drugs supported our
diagnosis, as well.
To our knowledge, this is the first report of AIH
induced by albendazole. We speculate that drug-induced AIH may be
prevented by avoiding use of drugs which have previously caused
hepatotoxicity in a given patient.
References
1. Watkins PB, Seeff LB. Drug-induced liver injury:
Summary of a single topic clinical research conference. Hepatology.
2006;43:618-31.
2. Björnsson E, Talwalkar J, Treeprasertsuk S, Neuhauser
M, Lindor K. Drug induced autoimmunehepatitis: Clinical characteristics
and prognosis. Hepatology. 2010;51: 2040-8.
|
|
|
 |
|

