|
|
|
Indian Pediatr 2015;52:
65-66 |
 |
Malignant Rhabdoid Tumor of Kidney and Brain
in an Infant
|
|
Deepti Shukla, *Aditya Pradhan, Mukesh Bhardwaj and
Veena Malhotra
From the Departments of Histopathology and *Urosurgery,
BLK Hospital, Pusa Road, New Delhi, India.
Correspondence to: Dr Veena Malhotra, Histopathology
Department, BLK Hospital, Pusa Road, New Delhi, India.
Email:
veena.malhotra@blkhospital.com
Received: June 24, 2014;
Initial review: July 31, 2014;
Accepted: October 09, 2014.
|
|
Background: Malignant rhabdoid
tumors of kidney are associated with atypical teratoid rhabdoid tumors
of brain, both being characterized genetically by deletion/ mutation of
SMAR CBI/ INI gene. Case characteristics: 6-month-old male
presented with a brain tumor and was subsequently found to have
malignant rhabdoid tumor of kidney. Interventions: Surgical
resection of brain tumor followed by chemotherapy and subsequently
resection of renal tumor. Outcome: Child died seven months after
initial presentation. Message: Children presenting with embroynal
brain tumor, should be investigated for renal tumors and vice versa.
Keywords: Hematuria, Kidney, Mutation, Tumor.
|
|
Malignant rhabdoid tumor of kidney is highly
aggressive tumor of infancy and childhood. Concomitant brain tumor is
present in almost 21% of patients [1]. These tumors are characterized
genetically by deletion/ mutation of SMARCBI/INI gene located on
chromosome 22q 11.2 [1]. We
describe a case of malignant rhabdoid tumor if kidney, who first
presented as posterior fossa brain tumor. Renal tumor was diagnosed only
when the child developed hematuria.
Case Report
A 6-month-old male child presented with history of
vomiting and increasing head size. Computerized tomography (CT) and
Magnetic resonance imaging (MRI) revealed a large heterogeneously
enhancing solid, cystic space occupying lesion with perilesional edema
and obstructive hydrocephalous. Ventriculoperitoneal shunt was done
followed by subtotal gross total excision of the tumor.
Histopathological examination revealed a neoplasm made up of sheets of
round, oval and spindle shaped cells. Cells had hyperchromatic nucleus
and moderate amount of lightly stained cytoplasm. Nuclear pleomorphism
and brisk mitosis was seen (Fig. 1). Typical rhabdoid
cells were not seen. Tumor cells showed positive staining for vimentin,
Epithelial Membrane Antigen (EMA) and Synaptophysin. They were negative
for Glial fibrillary acidic protein. A diagnosis of high grade malignant
embryonal tumor was given.
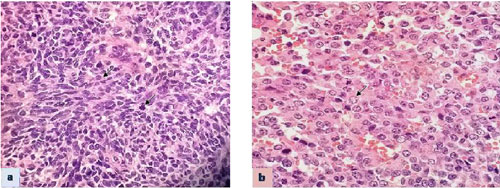 |
|
Fig. 1 Brain tumor showing medium
sized round to oval tumor cells with hyper chromatic nucleus,
moderate amount of cytoplasm and mitosis (arrow) H&E, x600 (a);
and Kidney tumor showing sheets of cells with nucleus showing
nucleolus and eosinophilic cytoplasm (arrow) H&E, x 600 (b).
(See website for color image).
|
Patient received four cycles of chemotherapy (Cisplatin,
Cyclophosphamide, Bleomycin and etoposide). Post four cycles of
chemotherapy, MRI brain showed progressive disease. Thereafter he
received two cycles of 2nd
line chemotherapy with Vinblastin plus ifosfamide plus cesplatin (VELP)
regimen. Subsequently child developed hematuria. Contrast enhanced
computerized tomography abdomen and chest showed a large heterogeneous
mass in the right kidney. Radical nephrectomy was done after 6 months of
initial presentation.
Right kidney measured 8×6.5×5.5 cm. Cut surface
showed a tumor replacing almost the entire kidney. Areas of hemorrhage
and necrosis were present. No capsular breach was seen. Microscopic
examination showed sheets of cells with nucleus showing central
nucleolus and eosinophilic cytoplasm. Mitosis and apoptosis was seen (Fig.
1). Areas of haemorrhage and necrosis were present. Lymphatic
invasion was seen. Tumor cells showed positive staining with vimentin
and EMA. Staining with INI-I on renal as well as earlier received CNS
tumor showed absence of nuclear staining.
Diagnosis of malignant rhabdoid tumor of kidney was
made. In view of INI-I negative staining in the CNS tumor as well, the
CNS tumor was also considered as atypical teratoid rhabdoid tumor
(AT/RT). As patient had already been treated with chemotherapy, patient
was counseled regarding the prognosis and managed by supportive
measures. Patient succumbed to his illness after one month of radical
nephrectomy.
Discussion
The exact cell type of derivation of Rhabdoid tumor
of kidney still remains unknown. Possible origin from primitive cells
located in renal medulla has been considered [2]. Rhabdoid tumor of
kidney is made up of cells arranged as diffuse sheets or as alveolar or
trabecular pattern. Extra renal rhabdoid tumors are being increasing
recognized [3].
Our case initially presented as CNS tumor. As
rhabdoid cells were not well-defined in the tumor, it was diagnosed as
high grade malignant embryonal tumor. Renal tumor was diagnosed only
when the child developed hematuria. In an infant or very young child
presenting with CNS tumor of ill-defined morphology, diagnosis of AT/RT
should be considered as rhabdoid cells may not be seen in all AT/RT
tumors. Staining with INI-I help in differentiating this tumor from
other poorly differentiated tumors such as primitive neuroectodermal
tumor. An evaluation for simultaneous presence of renal tumor should be
done in view of association of renal and CNS tumor. On the other hand in
children presenting with malignant rhabdoid tumor of kidney, brain CT
scan for CNS tumor should be mandatory.
The gene mutated or deleted in malignant rhabdoid
tumor of kidney is SMARCB1/ gene, also referred as SNF5 or
INI-I or BAF47 [4]. Inactivation of both copies of gene
leads to loss of protein expression in the nucleus, which can be
detected by immunohistochemistry for INI-I. Absence of INI-I is quite
specific for AT/ RT brain and malignant rhabdoid tumors of kidney.
Besides this soft tissue epithelioid sarcomas, renal medullary tumors,
few peripheral nerve sheath tumors and familial schwano-matosis can show
absence of nuclear staining [4]. However, these tumors can be
differentiated from malignant rhabdoid tumors on morphology. Strong
correlation is seen between loss of INI immunostaining and presence of
INI –I mutation [5]. Malignant rhabdoid tumors express many stem cell
associated transcription factors [6].
Histone deacetylase inhibitior, romidepsin
restores CDKNIC in rhabdoid tumor cells and may help in treatment
[7]. Pharmacological inhibition of fibroblastic growth factor receptors
FGFRs has been proposed as potential novel therapy for malignant
rhabdoid tumors [8]. Drugs which target cell cycle or epigenetic genes
and targeted therapy specific for rhabdoid tumor subset molecular
profiles may also be useful in treatment of rhabdoid tumors [9].
Rhabdoid tumors can occur sporadically or as part of
hereditary cancer syndrome known as Rhabdoid Tumor Predisposition
Syndrome. Prognosis of malignant rhabdoid tumor of kidney is related to
age at the time of diagnosis and stage of disease and not to the
location of tumor [1]. Survival at 4 years for infants under 6 months at
the age of diagnosis was 8.8% in comparison 41.1% survival for children
with age at diagnosis of 2 years [1].
Thus infants and very young children presenting with
kidney tumors should be investigated for synchronous presence of brain
tumor or vice versa.
Contributors: DS: Histopathology diagnosis of
brain tumor; AP: Surgical resection of kidney tumor; MB: Grossing of
specimen and prepration of manuscript; VM: Diagnosis of brain and kidney
tumor and prepration of manuscript.
Funding: None; Competing interests: None
stated.
References
1. Tomlinson GE, Breslow NE, Dome J, Guthrie KA,
NorkooL P, Li S, et al. Rhabdoid tumor of the kidney in National
Wilms tumor study: Age at diagnosis as a prognostic factor. J Clin Oncol.
2005;23:7641-5.
2. Juan Rosai. Urinary tract In: Rosai and Ackerman’s
Surgical Pathology, 9th edition Mosby St. Louis. Missouri, 2004. p.
1250-51.
3. Garces-Inigio EF, Leung R, Sebire NJ, McHugh K.
Extrarenal rhabdoid tumors outside the central nervous system in
infancy. Pediatr Radiol. 2009;39:817-22.
4. Robert CW, Biegel JA. The role of SMARCBI/INI1 in
the development of rhabdoid tumor. Cancer Biol Ther. 2009;8:412-6.
5. Wu x, Dagar V, Algar E, Muscat A, Bandopadhayay P,
Ashley D, et al. Rhabdoid tumor: a malignancy of early childhood
with variable primary site, histology and clinical behavior. Pathology.
2008;40:664-70.
6. Deisch J. Raisanen J. Rakheja D.
Immunohistochemical expression of embryonic stem cell markers in
malignant rhabdoid tumors. Pediatr Dev Pathol. 2011;14:353-9.
7. Algar EM, Muscat A, Dagar V, Rickert C, Chow CW,
Biegel JA, et al. Imprinted CDKNIC is a tumor suppressor in
rhabdoid tumor and activated by restoration of SMARCB1 and histone
deacetylase inhibitors. PLoS One. 2009;4:e4482.
8. Wohrle S, Weiss A, Ito M, Kauffman A, Murakami M,
Jagani Z, et al. Fibroblast growth factor receptors as novel
therapeutic targets in SNF5- detected malignant rhabdoid tumors. PLOS
One. 2013;8:e77652.
9. Brisks DK, Donson AM, Patel PR, Sufit A, Algar EM,
Dunham C, et al. Pedratic rhabdoid tumors of kidney and brain
show many differences in gene expression but share dysgrgulation of cell
cycle and epigenetic effector genes. Pediatr Blood Cancer.
2013;60:1095-102.
|
|
|
 |
|

