|
|
|
Indian Pediatr 2013;50:
127-133 |
 |
Clinical Profile of Interstitial Lung Disease
in Indian Children
|
|
Jhuma Sankar, Mrinal S Pillai, M Jeeva Sankar, Rakesh
Lodha and Sushil K Kabra
From the Department of Pediatrics, All India Institute
of Medical Sciences, New Delhi, India
Correspondence to: Dr Sushil K Kabra, Professor,
Department of Pediatrics, All India Institute of Medical Sciences, New
Delhi, India. [email protected]
Received: December 27, 2011;
Initial review: January 20, 2012;
Accepted: March 30, 2012.
Published online: June 10, 2012.
PII: S097475591101056-1
|
Objective:
To describe the
clinical spectrum and factors associated with poor short-term outcomes
in children with interstitial lung disease (ILD).
Design: Retrospective chart review
Setting: Pediatric Chest Clinic of a tertiary
care hospital
Methodology: We retrieved information regarding
clinical course and laboratory features of all children diagnosed as ILD
between January 1999 and February 2010. Disease severity was assessed
using ILD score based on clinical features and SpO2 at the time of
initial evaluation. Outcome was assessed after 3 months of initial
diagnosis as improved or death/no improvement in symptoms.
Results: 90 children (median age, 6.8 years; 62%
boys) were diagnosed to have ILD during this period. 46 children were
classified as having ‘definite ILD’ while 44 had ‘possible ILD’. The
commonest clinical features at presentation were cough (82.2%), dyspnea
(80%), pallor (50%), and crackles (45.6%). 3 children (3.3%) died while
21 (23%) showed no improvement in clinical status on follow-up at 3
months. A higher ILD score (RR 3.72, 95% CI 1.4, 9.9) and lower alkaline
phosphatase levels (median [IQR]: 205 [175.2] vs. 360 [245.7]; P=0.006)
were found to be significantly associated with worse outcomes.
Conclusion: The common clinical features of ILD
in our study included breathlessness, cough and hypoxemia. A working
diagnosis of ILD can be made with the help of imaging, bronchoscopy, or
lung biopsy. A simple score based on clinical findings and pulse-oximetry
might predict those children with poor short-term outcome.
Key words: ILD; Interstitial lung disease; ILD score; Lung
biopsy.
|
|
T he term interstitial lung
disease (ILD) encompasses heterogeneous lung conditions with a common
denominator of disordered gas exchange and diffuse infiltrates on X-ray
[1]. The exact incidence of childhood ILD is unknown. A 3-year survey of
chronic ILD in immunocompetent children in the United Kingdom and
Ireland has reported the prevalence to be 3.6 per million children [2].
Diagnosis of ILD is confirmed with the help of noninvasive and invasive
tests. Although lung biopsy is considered to be the gold standard for
diagnosis of ILD, its role in every patient of ILD is being questioned
by both adult and pediatric pulmonologists alike, and using a systematic
approach to diagnosis is being suggested as the way forward in these
patients [3-4].
The outcome of children with ILD in terms of death-
and disease-free survival is reported to be 15- 60% [5-8] and 50%,
respectively [8]. The available data on the clinical profile of children
with ILD mostly come from small case series that included less than 30
children [5-11]. Also, many of these reports [6-9] had focused on one or
more specific conditions such as fibrosing alveolitis or desquamative
interstitial pneumonitis (DIP) rather than looking at the complete
spectrum of ILD. Only one study, published in the late 1990 [5], has so
far reported the factors influencing outcomes in these children. The
objective of this study was therefore to evaluate the clinical profile
of children diagnosed to have ILD by noninvasive and/or invasive tests,
and to determine the factors associated with poor outcomes in them.
Methods
We conducted a retrospective chart review of children
who were diagnosed to have ILD between January 1999 and March 2010. The
diagnosis of ILD was made in the presence of progressive/persistent
respiratory distress with duration of illness of at least one month,
hypoxemia (documented by oxygen saturation), diffuse bilateral
infiltrates on chest X-ray and/or characteristic findings in high
resolution computed tomography (HRCT) with or without lung biopsy
findings suggestive of ILD [5]. Children with underlying congenital
heart disease, bronchopulmonary dysplasia (BPD), cystic fibrosis,
malignancy, primary or acquired immunodeficiency, coagulation disorders,
vasculitis, pulmonary tuber-culosis, celiac disease and vascular
malformations were excluded from the study.
We primarily categorized these children into two
major groups - ‘definite ILD’ and ‘possible ILD’ - based on their
clinical features, results of noninvasive tests such as X-ray and
HRCT, and results of invasive tests like bronchoscopy and biopsy. The
definitions used to classify these patients into definite ILD and
possible ILD are provided in Box I.
Hospital case records of children diagnosed as ILD
were retrieved for collection of data regarding the clinical course,
laboratory investigations such as HRCT chest (findings such as
geographical hyperlucency, septal thickening, ground glass opacity, lung
consolidation, and cysts and nodules), bronchospcopy and bronchoalveolar
lavage (BAL) analysis etc. Information on the treatment received
including steroids, immunosuppressive agents, home oxygen therapy and
the follow-up data of these children were also retrieved from the
records.
For assessing the disease severity, we assigned an
illness score originally proposed by Fan, et al. [12] based on
information from the patient records at the time of their initial
evaluation. We scored the patients from 1 to 5 based on increasing
severity of illness; accordingly, patients were given a score of 1 if
they were asymptomatic; 2, if symptomatic with normal room air
saturations; 3, if symptomatic with abnormal saturation/cyanosis during
exercise; 4, if symptomatic with abnormal room air saturation/cyanosis
at rest; and 5, if they were symptomatic with clinical and
echocardiographic features of pulmonary hypertension.
Outcomes: The short-term outcomes assessed
were death and symptomatic improvement at follow up from after 3 months
of starting therapy till the time of last follow up record available. By
symptomatic improvement we mean improvement in dyspnea, hypoxemia and/or
lung function tests. We also evaluated the determinants of poor outcomes
(death or no symptomatic improvement) such as age, gender, duration of
symptoms prior to presentation, effect of severe malnutrition (defined
as grade 3 and 4 protein energy malnutrition (PEM) according to Indian
Academy of Pediatrics (IAP) classification for malnutrition [13]),
common signs and symptoms at presentation (such as cough, dyspnea,
hemoptysis, pallor, clubbing, crackles and murmur), hematological
investigations at presentation such as total leucocyte count, liver
function tests at presentation such as serum glutamic oxalacetic
transaminase (SGOT), serum glutamic pyruvic transaminase (SGPT),
Alkaline Phosphatase (ALP), presence of abnormal chest X-ray and
HRCT findings on initial workup, bronchoscopy/ BAL findings and biopsy
at presentation(lung/liver, bone marrow, skin) suggestive of specific
disease.
Data were collected using a predesigned performa and
entered in Microsoft Excel 2003. Statistical analysis was done using
Stata 9.1 (StataCorp, College Station, TX). Data are presented as mean
(SD) or number (%) as appropriate. We compared the categorical variables
between the groups (ILD scores of <3 vs. scores of >3;
improved vs. not improved/died) using Fisher’s exact test (if the
expected number in any cell of the 2×2 table was <5) or Chi-square test.
The continuous variables in these groups were compared using independent
Student’s t-test (for variables that were normally distributed) or
Wilcoxon rank-sum test (for variables that were not normally
distributed).
Results
We reviewed the records of 2017 children; 90 were
diagnosed to have ILD (46 definite ILD, 44 possible ILD). The median
(IQR) age of these children was 6.8 (3,10) years. The youngest child was
7 months and the oldest 17 years of age. Diagnosis was confirmed on
histopathology in 14 (15.5%) cases based on clinical features and
bronchoalveolar lavage findings alone in 26 (28.9%) patients, and on
clinical grounds alone in 6 (6.7%) patients with hypersensitivity
pneumonitis.
The age of onset of symptoms in most of the children
(n=73; 81.1%) was beyond infancy (>1 year). The median duration
of symptoms at presentation was 12 months (IQR: 5-36 months). A family
history of similar illness and history of exposure to radiation, drugs
or chemicals were elicited in eight children each. Table 1
lists the clinical and laboratory features of children with ILD.
TABLE I Diagnostic Subgroups in Patients with ILD
| |
N=90
|
Died |
|
|
(n=3) |
|
Definite ILD |
n=46 (51.1) |
|
|
Langerhans cell histiocytosis
|
10 (21.7) |
0 |
|
Desquamative interstitial pneumonitis
|
1 (2.1) |
0 |
|
Systemic lupus erythematosus
|
1 (2.1) |
0 |
|
Idiopathic pulmonary hemosiderosis
|
26 (56.5) |
0 |
|
Hypersensitivity pneumonitis
|
6 (13) |
0 |
|
Pulmonary alveolar proteinosis
|
1 (2.1) |
0 |
|
Pulmonary microlithiasis
|
1 (2.1) |
0 |
|
Possible ILD |
n=44 (48.8) |
|
|
Idiopathic pulmonary hemosiderosis
|
10 (22.7) |
1 |
|
Sarcoidosis
|
2 (4.5) |
0 |
|
SJ syndrome associated
|
5 (11.3) |
0 |
|
BOO pneumonia
|
3 (6.8) |
0 |
|
Post infectious (tuberculosis/measles)
|
8 (18.1) |
0 |
|
Radiation pneumonitis
|
1 (2.3) |
0 |
|
Unclassified ILD
|
15 (34) |
2 |
|
Data represented as number (%); ILD, Interstitial lung disease;
SJ: Steven Johnson syndrome; BOO: Bronchiolitis Obliterans
Organizing.
|
TABLE II Association Between ILD Scores at Admission and Various Parameters
|
Parameters |
ILD score ≥3 (n=53) |
ILD score 2 or less (n=37) |
P
|
|
ILD score [Median (IQR)] |
2(2, 2) |
3(3, 4) |
|
|
Age (mo) [Median (IQR)]
|
96(58, 120) |
72(30, 196) |
0.06# |
|
Duration of symptoms (mo) [Median (IQR)]
|
13(5, 42) |
12(6, 30) |
0.81# |
|
Recurrent respiratory infections |
30(56.6) |
19(51.3) |
0.31 |
|
Severe malnutrition (PEM grade 3-4)* |
9(17) |
2(5.4) |
0.1 |
|
Symptoms and signs |
|
|
|
|
Cough |
45(84.9) |
29(78.4) |
0.44 |
|
Dyspnea |
46(86.8) |
26(70.3) |
0.03 |
|
Hemoptysis |
21(39.6) |
10(27.3) |
0.22 |
|
Pallor |
25(47.2) |
16(43.2) |
0.72 |
|
Clubbing |
32(60.4) |
1(2.7) |
<0.001 |
|
Crepitation |
26(49) |
15(40.5) |
0.43 |
|
Murmur |
2(3.8) |
1(2.7) |
1.0 |
|
Diagnostic subgroups |
|
|
0.49 |
|
Definite ILD |
25(54.4) |
21(45.7) |
|
|
Possible ILD |
28(63.6) |
16(36.4) |
|
|
Diagnosis |
|
|
0.17 |
|
IPH |
19(35.85) |
16(43.2) |
|
|
LCH |
3(5.7) |
7(18.9) |
|
|
Others
|
19(35.9) |
10(27) |
|
|
Unclassified ILD |
11(20.7) |
4(10.8) |
|
|
DIP |
1(1.9)) |
0 |
|
|
Outcome |
|
|
0.008 |
|
Died |
2(3.8) |
1(2.7) |
|
|
Improved activity by 3 months |
27(50.9) |
31(83.8) |
|
|
No improvement by 3 months |
18(34) |
3(8.1) |
|
|
Lost to follow up |
6(11) |
2(5.4) |
|
|
Data represented as number (%) unless specified otherwise;
PEM, Protein energy malnutrition; ILD, interstitial lung
disease; LCH, Langerhans cell histiocytosis; DIP, desquamative
interstitial pneumonitis; # analysis done using Wilcoxon rank
sum test. |
Laboratory findings: Of the 45 children with
clinical pallor, 23 (56%) had hemoglobin levels of 8 g/dL or less and 8
(19.5%) had hemoglobin of 4 g/dL or less. The other relevant
investigations are listed in Web Table 1. A restrictive
pattern of lung disease was found in 30 of the 42 children (71%) whose
spirometry results were available. The bronchoscopy findings were
available in 53 (58.9%) of the case records. The bronchoalveolar lavage
(BAL) was positive for hemosiderin-laden macrophages in 26 (49%)
children, who were then diagnosed to have idiopathic pulmonary
hemorrhage (IPH). PAS positive macrophages were seen in 4 (7.5%)
children – one each had pulmonary alveolar proteinosis and pulmonary
alveolar microlithiasis while the other two were diagnosed to have LCH
based on tissue biopsy findings. Lung biopsy report was available in 4
(4.4%) cases with findings such as hemosiderin-laden macrophages (n=2),
histiocytes with S-100 positivity (n=1), and diffuse type II
pneumocyte hyperplasia (n=1). Majority of the reports (n=10)
of other tissue biopsies confirmed the diagnosis of LCH (S-100 and CD8
positive cells).
Diagnostic subgroups: The most common diagnoses
made were IPH, LCH and unclassified ILD (Table I). A
diagnosis of IPH was made on the basis of strong clinical suspicion of
IPH corroborated with findings of BAL analysis showing hemosiderin laden
macrophages. A diagnosis of LCH was made based on clinical features, BAL
analysis showing histiocytes/lipid laden macrophages and tissue biopsy
findings (lung or otherwise) positive for histiocytes with S-100
positivity. Almost 2/3 rd (n=56;
62.2%) of the children were investigated for tuberculosis prior to
presentation and 15 (16.7%) were already on anti-tuberculous therapy. In
all of them, work up for tuberculosis was inconclusive. Work-up for
autoimmune disease - ANA (anti-nuclear antibody), perinuclear component
of antineutrophil cytoplasmic antibody (p-ANCA) was available in 34
children (37.8%); it was negative in all except for one child with SLE.
Hypersensitivity to cow’s milk protein (Heiner’s syndrome) was suspected
in 4 patients with IPH; however, antibodies to cow’s milk protein was
negative in all of them and none improved clinically on milk-free diet.
Hypersensitivity pneumonitis was diagnosed in 6 children with definite
history of exposure to bird droppings, feathers, air cooler mist, paint
and plastics. Three of them (aged 12-14 years) were working in paint and
plastic manufacturing units. They improved with removal of the offending
agents from their environment.
A definite diagnosis of Steven Johnson syndrome (SJS)
prior to the onset of symptoms of ILD was forthcoming in five patients
with the disease. A diagnosis of sarcoidosis was considered in two
children with lymphadenopathy and hepatosplenomegaly by HRCT (hilar
lymphadenopathy) and elevated angiotensin converting enzyme (ACE)
levels. Bronchiolitis obliterans organizing pneumonia (BOOP) was
suspected in 3 patients with HRCT showing geographical hyperlucency. One
patient with Hodgkin’s lymphoma who had received radiotherapy for 6
months presented with features of ILD within 11 months of starting
therapy. Two patients had a definite history of measles several months
before the onset of symptoms but as none of the investigations were
conclusive, they were diagnosed as post measles ILD. Testing for measles
or tubercular antibodies was not possible in any of the patients due to
logistic reasons. In the remaining patients, there was a strong clinical
suspicion of ILD which could not be corroborated with either
radiological findings or bronchoscopy alone and there were no records of
biopsy available in them. They were therefore grouped under unclassified
disease.
ILD scores: Majority of the children (53; 58.9%)
had an ILD score of ³3.
We chose this cut-off (³3)
based on the area under curve in the receiver operating characteristics
(ROC) curves - the area under the ROC curve for this score was 0.72 (95%
CI: 0.61-0.84). This cut-off was used to distinguish between severe and
mild disease.
Of the baseline variables, only clubbing was found to
be significantly associated with higher ILD scores. The severity score
was comparable between the two major diagnostic subgroups- definite ILD
and possible ILD (Table II).
Treatment: Almost all (87; 96.7%) the children
received steroids either in oral (79; 90.8%) or inhaled (8; 9.2%) form.
The indication for steroid therapy was symptomatic disease irrespective
of the saturations. Oral and inhaled steroids were given depending on
the clinical manifestations. The dose for oral steroids was 1-2
mg/kg/day for a minimum period of 6-8 weeks depending on the patients’
symptomatology. In addition to steroids, 44 children (54.3%) received
hydroxychloroquine and 7 (7.7%) children received azathioprine.
Hydroxy-chloroquine was given as a first line agent in patients of IPH
along with steroids or was used as a second line agent like azathioprine
in those not responding to steroids alone. Children with LCH were
prescribed chemotherapy as per protocol. Majority (58; 64.4%) of the
children showed complete remission of symptoms after 3 months of
initiation of therapy.
Outcome: Of the 90 children, only 3 died (two of
them had unclassified ILD and one had IPH), while the rest were
discharged. Eight children (9.1%) were lost to follow-up at the first
review period, i.e. at 3 months. The median duration of follow-up in the
rest of the survivors was 9 months (IQR 6 to 18.5 months). The duration
of follow up was longer in the patients with definite ILD (18.5 months,
IQR 6, 22) as compared to those with possible ILD (7.5 months, IQR 5,
15). The frequency and duration of follow-up was decided based on the
ongoing symptoms/signs and the response to the treatment.
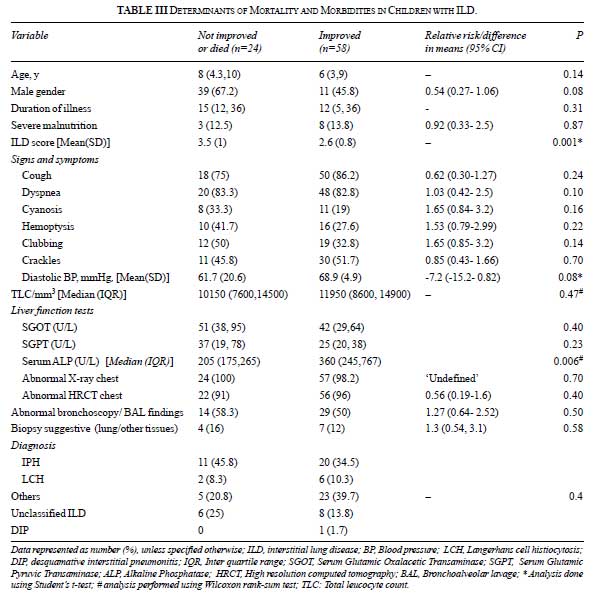
On univariate analysis, we found two factors to be
significantly associated with poor outcome– high ILD scores at initial
evaluation and lower alkaline phosphatase levels (Table III).
The group which showed no/partial improvement required recurrent
admissions, blood transfusions, home oxygen therapy, hydroxychloroquine
and had longer follow-up.
Discussion
The main aim of presenting our data is to provide
clinical details and short term outcome of patients diagnosed as ILD on
the basis of history, examination and limited investigations available.
At present, in resource limited settings, diagnosis of ILD is rarely
made as in most of the circumstances lung biopsy is not available and
these children receive inappropriate treatment (antibiotics,
antitubercular drugs, etc).
Lung biopsy is considered as gold standard for
diagnosis of ILD however, getting a lung biopsy in children is difficult
especially when they present in advanced stage of illness and have a
very high risk for anesthesia. In addition, biopsy may not always be
conclusive [4,5,14]. Of late, the trend is shifting towards a systematic
approach to the diagnosis of patients with ILD rather than subjecting
every patient to biopsy. Lung biopsy could possibly be reserved for
those children in whom the diagnosis is inconclusive even after
noninvasive tests and/or there is poor response to therapy. In children
suspected to have ILD secondary to systemic disorders such as LCH,
sarcoidosis etc., a tissue biopsy of the other affected tissues should
suffice.
The clinical profile, radiological features,
pulmonary function test results, bronchoscopy findings and tissue biopsy
reports were comparable with previous studies from developed as well as
developing countries [5,15-17]. The only difference was in the
diagnostic yield of HRCT. The diagnostic yield of HRCT was higher in our
study (92%) in suspected cases as compared to the study by Copley, et
al. [18] in which only 66% of HRCT chest was suggestive of
diagnosis. Increased awareness of the disease with time and knowledge of
specific CT features suggestive of ILD [18] could have played a role in
the increased yield. While previous studies have reported the commonest
anomaly on HRCT as ground glass appearance [16,18-19] we found septal
thickening to be as common. The large number of children with IPH and
LCH in our study could have resulted in this finding. Bronchoalveolar
lavage proved to be a very useful tool in the diagnosis of patients with
alveolar hemorrhage and pulmonary alveolar proteinosis in contrast to a
previous study from our country where the BAL showed only neutrophils
and did not contribute much to the diagnosis [17].
IPH and unclassified ILD emerged as the most common
diagnostic subgroups in our study. This was followed closely by patients
with LCH and post infectious ILD. Findings of our study are in agreement
with those of Fan et al who reported no specific diagnoses in 19 of
their 99 patients despite a complete diagnostic evaluation [5].
Infection associated ILD and pulmonary vascular disorders were the other
common diagnostic subgroups reported [5].
Three children died in our study within three months
of diagnosis. In view of the retrospective nature of the study and the
numbers lost to follow up, we could not precisely estimate the number of
children who died after the last documented follow up dates. Fan, et
al. [5] had reported 15 deaths (15%) out of a cohort comprising 99
patients. Several authors have reported mortality ranging from 15-60% in
children with ILD [5,6] and these figures reached 100% in children with
specific disorders such as diffuse developmental disorders and abnormal
surfactant function [5,8]. We could not establish the histopathologic
diagnosis in the three children who died. The only child with DIP
survived. The final diagnosis, relatively short follow up duration
available from the records and the numbers lost to follow up could have
contributed to these differential results in our study.
Similar to findings of Fan, et al. we observed
that a higher severity of illness score calculated at admission
correlated with poor outcomes. As there were only 3 deaths and none of
the diagnostic subgroups dominated the worse outcome group, we did not
have to control for any diagnostic categories. Delayed diagnosis of the
condition due to the disease mimicking a number of common disorders,
particularly tuberculosis in our country is the most probable
explanation for these high scores in the study population.
In addition to the ILD score, the median serum
alkaline phosphatase levels were found to be higher in the group with
better outcome. This could be explained by the high number of patients
with LCH with multisystem disease in this group. Paradoxically patients
with LCH as such did not have significant bearing on the outcomes.
Therefore, it is difficult to explain the association. We could not find
any previous studies showing similar association. None of the other
factors associated with the disease such as age of onset, gender,
duration of illness, clinical signs and symptoms or investigations had
any bearing on the outcomes.
The major limitations of our study are its
retrospective nature and lack of histopathological confirmation of the
disease in most patients. In addition, though the numbers were
reasonable considering the rare nature of the disease, it might not have
been adequately powered in detecting all the factors influencing the
outcomes.
To conclude, in any child with a long drawn history
of respiratory symptoms not suggestive of the common infectious or
non-infectious conditions in that set up, a possibility of ILD should be
strongly considered and confirmed with necessary investigations. With
our report we want to raise awareness about ILD among pediatricians so
that diagnosis can be made in the initial stages by using HRCT of chest,
bronchoalveolar lavage (BAL) and other less invasive procedures and
appropriate treatment can be instituted.
Contributors: SKK: conceived and designed the
study and revised the manuscript for important intellectual content. He
will act as guarantor of the study; JS: conducted the study, analyzed
the data and drafted the paper; MP helped in data collection, analysis
and in revising the manuscript. MJS and RL: provided inputs regarding
the design and revised the manuscript for intellectual content. The
final manuscript was approved by all authors.
Funding: None; Competing interests: None
stated.
|
What is Already Known?
• ILD is a rare pulmonary disorder of
childhood with diverse etiology.
• Lung biopsy is the gold standard for
diagnosis of ILD.
What This Study Adds?
• In settings where lung biopsy is not
feasible in children suspected to have ILD, the diagnosis of ILD
may be made based on the HRCT and bronchoscopy findings.
• ILD scores of 3 or more may predict poor outcome in these
children.
|
References
1. Clement A, Nathan N, Epaud R, Fauroux B, Corvol H.
Interstitial lung diseases in children. Orphanet J Rare Dis. 2010;5:22.
2. Dinwiddie R, Sharief N, Crawford O. Idiopathic
interstitial pneumonitis in children: a national survey in the United
Kingdom and Ireland. Pediatr Pulmonol. 2002;34: 23-9.
3. Raghu G. Interstitial lung disease: a diagnostic
approach. Are CT scan and lung biopsy indicated in every patient? Am J
Respir Crit Care Med. 1995;151:909-14.
4. Fan LL, Kozinetz CA, Deterding RR, Brugman SM.
Evaluation of a diagnostic approach to pediatric interstitial lung
disease. Pediatrics. 1998;101:82-5.
5. Fan LL, Kozinetz CA. Factors influencing survival
in children with chronic interstitial lung disease. Am J Respir Crit
Care Med. 1997;156:939-42.
6. Stillwell PC, Norris DG, O’Connell EJ, Rosenow EC
3rd, Weiland LH, Harrison EG Jr. Desquamative interstitial pneumonitis
in children. Chest. 1980; 77: 165-71.
7. Diaz RP, Bowman CM. Childhood interstitial lung
disease. Semin Respir Med. 1990; 11: 253-68.
8. Deutsch GH, Young LR, Deterding RR, Fan LL, et
al. ChILD Research Co-operative. Diffuse lung disease in young
children: application of a novel classification scheme. Am J Respir Crit
Care Med. 2007;176:1120-8.
9. Sharief N, Crawford OF, Dinwiddie R. Fibrosing
alveolitis and desquamative interstitial pneumonitis. Pediatr Pulmonol.
1994;17:359-65.
10. Osika E, Muller MH, Boccon-Gibod L, Fauroux B,
Sardet A, Grosskopf C, Couvreur J, et al. Idiopathic pulmonary
fibrosis in infants. Pediatr Pulmonol. 1997; 23: 49-54.
11. Paiva MA, Amaral SM. Chronic interstitial lung
disease in children. J Pediatr (Rio J). 2007; 83: 233-40.
12. Fan LL, Mullen AL, Brugman SM, Inscore SC, Parks
DP, White CW. Clinical spectrum of chronic interstitial lung disease in
children. J Pediatr. 1992;121:867-72.
13. Shah PM. Report of Nutrition Sub-committee of
Indian Academy of Pediatrics. Indian Pediatr. 1972;9:360.
14. Fan LL, Langston C. Chronic interstitial lung
disease in children. Pediatr Pulmonol. 1993;16:184-96.
15. Coren ME, Nicholson AG, Goldstraw P, Rosenthal M,
Bush A. Open lung biopsy for diffuse interstitial lung disease in
children. Eur Respir J. 1999;14:817-21.
16. Clement A: Task force on chronic interstitial
lung disease in immunocompetent children. Eur Respir J. 2004;24: 686-97.
17. Vijayasekaran D, Giridhar S, Gowrishankar NC,
Nedunchelian K, Senguttuvan M. Pediatric interstitial lung disease.
Indian Pediatr. 2006;43:899-903.
18. Copley SJ, Coren M, Nicholson AG, Rubens MB, Bush
A, Hansell DM. Diagnostic accuracy of thin-section CT and chest
radiography of pediatric interstitial lung disease. AJR Am J Roentgenol.
2000;174:549-54.
19. Vrielynck S, Mamou-Mani T, Emond S, Scheinmann P,
Brunelle F, de Blic J. Diagnostic value of high-resolution CT in the
evaluation of chronic infiltrative lung disease in children. AJR Am J
Roentgenol. 2008;191:914-20.
|
|
|
 |
|

