|
|
|
Indian Pediatr 2013;50: 119-126
|
 |
Diagnosis and Management of Gastroesophageal
Reflux Disease (GERD): An Indian Perspective
|
|
Ujjal Poddar
From the Department of Pediatric
Gastroenterology, Sanjay Gandhi Postgraduate Institute of
Medical Sciences, Lucknow,
Uttar Pradesh, India.
Correspondence to: Dr Ujjal Poddar, Additional
Professor, Department of Pediatric Gastroenterology, SGPGIMS,
Lucknow 226 014, Uttar Pradesh.
Email:
[email protected]
|
Need and Purpose: The scarcity of literature and lack of published
guidelines on gastroesophageal reflux disease (GERD) from India, have
necessitated this review.
Methods: A literature search in PubMed was
conducted with regard to epidemiology, clinical features, investigation
and management of GERD in children. English language studies published
full over the last 20 years were considered and relevant information was
extracted.
Results: Nearly half of all healthy babies
regurgitate at least once a day by 4 months of age and this subsides in
90% of them by 1 year. In contrast, GERD prevalence increases with age
and by adolescence it is similar to adults (20%). While regurgitation in
infancy does not need investigation or therapy, ‘empirical’ proton pump
inhibitor (PPI) for 4 weeks is justified in older children with
classical GERD symptoms. There is no gold-standard investigation for
GERD. A pH study with or without impedance is useful in extraesophageal
manifestations and endoscopy in esophagitis. Proton pump inhibitors
(PPI) play a pivotal role in the management of GERD and its long-term
use has been shown to be safe in children. Antireflux surgery plays a
minor role due to, its associated morbidity and high failure rate,
especially in the high risk group who needs it most.
Conclusions: Regurgitation in infancy need not be
investigated unless there are warning features. Empirical PPI therapy is
justified in older children and adolescents with typical reflux
symptoms. pH study in extraesophageal manifestations and endoscopy for
esophagitis are the investigations of choice. PPI is the mainstay of
therapy in GERD.
Key words: Endoscopy, Impedance, pH study, Proton pump
inhibitors, Regurgitation.
|
|
G
astroesophageal reflux or
GER means involuntary passage of gastric
contents into the esophagus and is often
physiological but gastroesophageal reflux
disease or GERD means symptoms or complications
associated with pathological GER [1].
Prevalence
GER or regurgitation is very
common in infancy, both in the West as well as
in India. In a study in 948 infants <13 months
age from USA [2], it was shown that at least one
bout of regurgitation per day was present in 50%
of babies between 0 to 3 months of age and this
figure was 67% at 4-6 months of age but after
that there was a sharp decline to 21% at 7-9
months of age and by 10-12 months only 5% babies
continued to have regurgitation. Though the
prevalence of more significant regurgitation ( ³4
times/day) was much less but babies with
significant regurgitation also followed a
similar pattern, 20% at 0-3 months, 23% at 4-6
months and only 3% at 7-9 months and by 12
months just 2% babies continued to have
significant regurgitation. In a similar study in
863 children, from Australia, the prevalence of
GER was 41% at 3-4 months and this became <5% at
13-14 months and negligible by 19 months of age
[3]. A recent study from Italy in 2642 patients
aged 0-12 months, showed a lower frequency of
infant regurgitation (12%) but the natural
history was similar (regurgitation subsided in
88% by 12 months and 100% by 24 months) [4]. On
the contrary, the prevalence of GERD in infancy
is just 5%-9% of all infants with regurgitation
[2, 5].
In an elegantly conducted
study from India in 602 children of 1-24 months
of age, De, et al.[5] showed that the
prevalence of regurgitation was 55% at 1-6
months age and it dropped to 15% at 7-12 months
of age and further reduced to 10% at 12-24
months of age. All these studies [2-5] suggest
that GER is frequently seen in early infancy and
it almost disappears by one year of age.
Persistence or appearance of regurgitation
beyond 18 months of age is suggestive of
pathological condition.
However, the prevalence of
GERD i.e. symptoms associated with GER is
uncommon in younger children. In the West, the
prevalence of GERD is almost 20% in the general
population [6]. In a study from USA [7]
involving 566 children between 3-9 years of age
(parental interview) and 615 children between
10-17 years (directly interviewed), pyrosis or
heartburn was reported in 1.8% of the 3-9 years
age group and 3.5% in the 10 to 17 years age
group compared to 22% in adults (>18 years).
Hence, the prevalence of GERD slowly increases
with age during childhood and becomes quite
frequent among young adults.
Presenting Symptoms
Presenting symptoms in
infants and children are different (Table
I) [8]. The majority of infants, who are
otherwise healthy, present with regurgitation or
vomiting with no failure to thrive or other
associated symptoms. These infants are labeled
as ‘happy spitters’. In infants with
regurgitation, it is important to differentiate
physiological GER from other causes of vomiting
and GERD (Table I and II). Infants
with GERD are associated with growth failure or
indirect symptoms of pain due to esophagitis
like irritability, feeding difficulty, sleeping
difficulties, crying episodes, anemia etc.
Rarely apnea or apparent life-threatening events
might be a consequence of GERD but their causal
relationship has not yet been established
convincingly. Chronic respiratory diseases and
upper airway problems like sinusitis, otitis
media, laryngitis, dental erosion etc. have been
described in infants with GERD but the causality
and temporal association of these extra-esophageal
symptoms have not yet been established [9]. In
children and adolescents, symptoms and
complications of GERD are similar to those in
adults. Commonest symptom in this group is
heartburn or substernal pain. Important aspects
of history which help in differentiating GERD
from other causes of vomiting are given in
Table II. A subset of children with
underlying disorders like mental retardation,
repaired tracheo-esophageal fistula and
esophageal atresia etc. are at higher risk of
developing severe GERD and listed in Table
III [6].
TABLE I Presenting Symptoms of Gastroesophageal Reflux Disease (GERD) in Infants and Children [8]
|
Infants |
Children |
|
Vomiting |
Regurgitation |
|
Poor weight gain |
Heartburn and retrosternal chest pain |
|
Irritability |
Dysphagia |
|
Feeding refusal or dysphagia |
Asthma or chronic cough |
|
Recurrent pneumonia |
Recurrent pneumonia |
|
Asthma and upper airway symptoms |
Anemia and hematemesis |
|
Apnea or apparent life-threatening event
(ALTE) |
|
TABLE II Important Aspects of History to Differentiate GER/GERD From Other Causes of Vomiting
|
• |
Vomiting |
Feeding history: Frequency and volume |
|
• |
Presence of bile |
Past medical history: neurological
disease, prematurity, history of aeso-digestive
surgery |
|
• |
Presence of blood |
|
|
• |
Presence of forceful emesis |
Family history: family history of reflux
and its severity |
|
• |
Frequency and amount of emesis |
|
|
• |
Presence of pain and irritability |
Medical history: drugs like
anticonvulsants, bronchodilators etc. |
|
• |
Associated constitutional symptoms |
|
|
• |
Other gastrointestinal symptoms |
|
TABLE III Conditions Predisposing to Severe Gastroesophageal Reflux Disease (GERD) in Children [6]
|
• |
Obesity |
|
• |
Neurological impairment like cerebral
palsy |
|
• |
Neuromuscular disease like congenital
myopathy |
|
• |
Genetic conditions like Trisomy 21 |
|
• |
Repaired trachea-esophageal fistula |
|
• |
Repaired esophageal atresia |
|
• |
Congenital diaphragmatic hernia |
|
• |
Chronic lung disease like
bronchopulmonary dysplasia,
bronchiectasis, asthma |
|
• |
Cystic fibrosis, scleroderma |
|
• |
Previous esophageal caustic injury |
|
• |
Significant prematurity |
|
• |
Strong family history of GERD, Barrett
esophagus or esophageal adenocarcinoma |
Diagnostic Approach to GERD
There is no gold standard for
the diagnosis of GERD. The choice of
investigation depends on the clinical situation
for which the investigation is asked for.
GERD in infants
The approach to infants is
illustrated in Fig. 1. In infants,
Orenstein’s infant GER questionnaire (i-GERQ) (Table
IV) [10] may help in distinguishing GER from
GERD. Similarly, Rome III criteria (Table
V) [11] can be used to diagnose GER in
infants. Orenstein, et al. have developed
a symptom-based 11 points questionnaire (I-GERQ
GERD) with maximum score of 25 to differentiate
GER from GERD and have shown that a score of >7
has 74% sensitivity and 94% specificity in
diagnosing GERD in infants. This questionnaire
was applied in Indian population [12] and has
shown to be easily adaptable and reproducible
but had lower diagnostic accuracy (sensitivity
of 43% and specificity of 79%) than the original
study. Nevertheless, I-GERQ GERD questionnaire,
because of its simplicity (takes just 20 minutes
to complete) and reproducibility, can be used to
segregate those infants who needs empirical
therapy or further investigation.
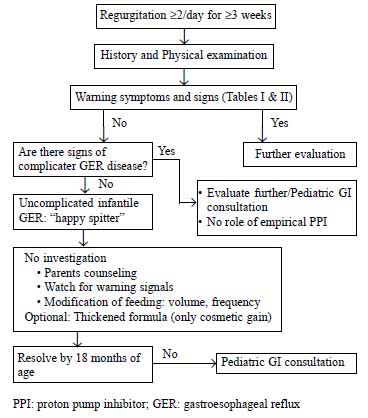 |
|
Fig.1 Suggested approach to
gastroesophageal reflux in infants [14].
|
TABLE IV Diagnostic Criteria of Infant Regurgitation According to the Rome III Classification [11]
|
Must include all of the following in
otherwise healthy infants 3 weeks to 12
months of age |
|
• |
Regurgitation 2 or more times per day
for 3 or more weeks |
|
• |
No retching, hematemesis, aspiration,
apnea, failure to thrive, feeding or
swallowing difficulties, or abnormal
posturing |
TABLE V GER vs. GERD in Infants. Modified Orenstein’s Infant GER Questionnaire [10].
|
Question |
Points |
|
1. |
How often does the baby usually spit up? |
|
|
•1 to 3 times per day |
1 |
|
•3 to 5 times per day |
2 |
|
•>5 times/day |
3 |
|
2. |
How much does the baby usually spit up? |
|
|
•1 teaspoonful to 1 tablespoonful |
1 |
|
•1 tablespoonful to 1 ounce |
2 |
|
•>1 ounce |
3 |
|
3. |
Does the spitting up seem to be
uncomfortable for the baby? |
2 |
|
4. |
Does the baby refuse feeding even when
hungry? |
1 |
|
5. |
Does the baby have trouble gaining
enough weight? |
1 |
|
6. |
Does the baby cry a lot during or after
feeding? |
3 |
|
7. |
Do you think the baby cries or fusses
more than normal? |
1 |
|
8. |
How many hours does the baby cry or fuss
each day? |
|
|
•1 to 3 hours |
1 |
|
•>3 hours |
2 |
|
9. |
Do you think the baby hiccups more than
most babies? |
1 |
|
10. |
Does the baby have spells of arching
back? |
2 |
|
11. |
Has the baby ever stopped breathing
while awake and struggling to breathe or
turn blue or purple? |
6 |
|
Maximum total score |
25 |
• Score >7, sensitivity: 74% and specificity: 94% for diagnosing GERD
GERD in children and
adolescents
The approach to older
children and adolescents is given in Fig.
2. In older children (> 8 years, who can
give proper history), history and physical
examination are the most important and the only
steps in most cases of GERD. In adults ‘empiric
therapy’ of PPI for 2 to 4 weeks is an accepted
method of diagnosing GERD with classical
symptoms of heartburn with or without
regurgitation [13]. Though there is no study of
empirical trial of PPI as a diagnostic test in
children, an empirical PPI trial of up to 4
weeks is justified in older children and
adolescents with classical symptoms of GERD
[14]. Diagnostic studies like endoscopy, pH
study, barium upper gastrointestinal series are
useful when symptoms are not classical and in
cases with complicated GERD. In a patient with
classical symptoms of GERD, there is no need to
confirm the presence of GER by pH study or by
endoscopy. However, in patients with extra-esophageal
symptoms like respiratory symptoms without any
GER symptoms, a pH study is required to document
reflux. Similarly, when esophagitis is suspected
(pain or blood loss) upper gastrointestinal
endoscopywith esophageal biopsy is recommended.
However, when there is any suggestion of an
anatomical abnormality like intestinal
obstruction or dysphagia, barium upper GI series
is indicated.
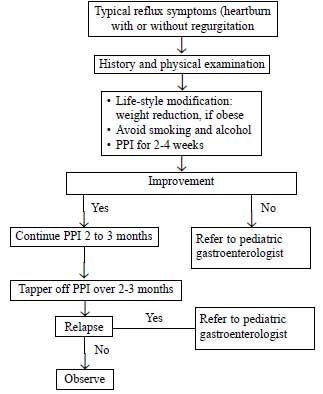 |
|
Fig.2 Suggested
approach to gastroesophageal reflux
disease in older children and adolescent
[14].
|
Esophageal pH-monitoring
24 hours ambulatory pH-metry
helps to establish the presence of acidic reflux
(pH < 4) in a patient who does not have GER
symptoms and it also helps to assess the
efficacy of medical therapy. The indications of
this test are; to quantify reflux in patients
with mainly extra-esophageal symptoms, to
measure GER in patients not responding to
antireflux treatment and in research. The
advantages of pH-metry are; it can be done in
any age (neonates to adults), it is relatively
non-invasive, but the main disadvantage is that
it does not measure non-acid or weakly acidic
reflux (pH ³4).
The main parameter in a pH-study, which helps in
diagnosing GERD, is the ‘reflux index’ (RI). RI
is the percentage of time esophageal pH is <4. A
RI >10% in infants and >5% in children are taken
as suggestive of GER [14, 15].
Multichannel Intraluminal-impedance (MII)
measurement
The basic principle of this
technique is to detect the change in electrical
resistance (or impedance) that occurs during the
passage of a bolus of gas or liquid across a
measuring segment (between electrodes) placed in
the esophagus. Impedance is inversely
proportional to electrical conductivity. Since
the conductivity of liquid (high) and air (low)
is different, MII can easily differentiate
liquid from gas reflux. Moreover, this study
detects both acid and non-acid reflux and the
direction of reflux (retrograde from stomach to
esophagus versus ante-grade bolus movement). The
combination of impedance with pH monitoring is
shown to be superior to pH-study alone for
evaluation of GER-related symptom association
[14]. Multichannel intraluminal impedance-pH
monitoring (MII-pH) has the advantage of picking
up acid, non-acid or weakly acid reflux, the
direction of reflux and also to distinguish
between liquid, solid and gas reflux in all age
groups[14-16].Indications of MII-pH study are
same as a pH-study [14]. The limitations of
MII-pH study are; high cost, limited
availability, limited therapeutic implications
(clinical relevance of measuring non-acidic
reflux remains doubtful) and the lack of
evidence-based parameters for assessment of GER
[16].
Endoscopy
Upper gastrointestinal
endoscopy is the best method of detecting
esophagitis as a consequence of GERD. However,
normal endoscopy (found in 60% to 80% cases of
GERD in children) [17] does not rule out GERD
and this type of GERD is called Non-erosive
reflux disease (NERD). Endoscopy needs to be
combined with a biopsy to increase the
diagnostic yield (especially in NERD) and to
rule out other causes of esophagitis (like
eosinophilic esophagitis, Crohn’s disease etc).
Indications of endoscopy are; persistence of
symptoms despite therapy, dysphagia or
odynophagia, evidence of GI bleeding or iron
deficiency anemia, stricture or ulcer on barium
study and long duration GERD to detect Barrett’s
esophagus. Advantages of endoscopy are; it gives
a direct information about the presence and
severity of esophagitis, detects complications
like ulcer, stricture, Barrett’s esophagus,
documents healing of erosive esophagitis after
therapy and endoscopic esophageal biopsy helps
to exclude other cause of esophagitis. Histology
is more sensitive than endoscopy in the early
stage (non-erosive stage). Erosive esophagitis
is the most definite evidence of GERD on
endoscopy. Hence, if there is no erosion or
mucosal break on endoscopy, biopsy (2 cm
proximal to gastroesophageal junction) helps to
establish the diagnosis of GERD. The most
important features of GERD on esophageal
histology are; basal zone hyperplasia (>20% of
total thickness) and elongation of papillae or
rete pegs (>50% of total thickness). Other
histological features are; infiltration with
neutrophils or eosinophils (<15/high power
field), growing of blood vessels in papilla etc
[18,19]. Nevertheless, recent studies have shown
that histological changes are neither sensitive
nor specific for reflux disease in NERD cases
and should not be used alone to diagnose or
exclude GERD [14].
Barium UGI series
This test is useful to detect
anatomical anomalies but is not useful in
diagnosing GERD. The sensitivity and specificity
of barium study to diagnose GERD is less than
50% [20,21]. It cannot differentiate
physiological from pathological reflux. Hence,
it is not recommended for the diagnosis of GERD
[14].
Nuclear scintigraphy
Technetium labeled milk scan
is a non-invasive test but has poor sensitivity
and specificity. The only situation where it may
be useful is recurrent pneumonia due to
aspiration of gastric contents. Retention of
radioactivity in lungs beyond 24 hours suggests
GERD as a cause. However, absence of
radioactivity in the lungs does not rule out
GERD. Nuclear scintigraphy is not recommended
for the routine evaluation of pediatric patients
with suspected GERD [14].
Management
GER in Infants (Happy
Spitters)
The most important part of
management is counseling. The natural history of
GER in infants needs to be explained to parents
or care-givers. Other measures are; feeding
advice, positioning and feed thickening. Mothers
should be instructed to avoid overfeeding,
forceful feeding, and to try to give small but
frequent feeds. pH studies have shown that
reflux is minimal in prone position but the risk
of SIDS is maximum in prone position and that’s
why prone position is not recommended in
infants. However, beyond infancy (>13 months)
left lateral position is found to be the best in
preventing reflux. Feed thickening by adding
rice, corn or potato starch decreases the number
regurgitation or vomiting but it does not
decreases the acid exposure of esophagus. Hence,
feed thickener has only cosmetic value but no
therapeutic benefit [22].In a subset of patients
(1-10%), regurgitation may be the manifestation
of Cow’s milk protein allergy (CMPA) [23]. If
there is no response to conventional therapy of
counseling, feed thickening in formula-fed
infants, a 2 to 4 weeks trial of hypoallergenic
milk (extensively hydrolyzed or amino acid
formula) is recommended and if the symptoms
subside then a challenge and continuation of
milk free diet is recommended. However, if there
is no response to hypoallergenic formula over 2
to 4 weeks then there is no point in continuing
the formula [14].
PPIs are not recommended in
this subset of patient as only a few of the
infants are likely to have acid-related cause
for their symptoms and the largest randomized,
controlled trial in infants showed that for
symptoms, presumably to be related to reflux
disease, a PPI was not better than placebo [24].
GERD in Children
Besides medication,
life-style modification in terms of weight
reduction, avoiding caffeine, chocolate,
abstinence from alcohol, tobacco helps in
children [14]. Adolescents, like in adults, may
benefit from the left lateral decubitus sleeping
position with head-end elevation.
Pharmacological therapy
Acid suppressants:
Children with GERD need potent acid suppression
therapy for at least 12 weeks. It has been shown
that proton pump inhibitors (PPIs) are more
potent and more effective than H 2-receptor
antagonists (H2RA).
Healing rate of erosive esophagitis with H2RA
like Ranitidine (6-8 mg/kg/day, BID or TID) or
Famotidine (1mg/kg/day, BID) is 60% to 70% and
with PPIs like omeprazole (0.7 to 3.5mg/kg/day,
OD) is 90% to 100% [25, 26]. Antacids can be
used for symptomatic relief for a brief period
but prolonged therapy is contraindicated in
children due to side effects.
Neutralizing or surface
protective agents (antacids or sucralfate):
Overall efficacy in relieving symptoms and
healing esophagitis of this group of drugs is
more than the placebo but less than H 2RA
or PPI. This group of drugs is useful for
symptomatic relief of heartburn but they should
not be used for long term therapy in children as
there is risk of aluminum toxicity (osteopenia,
rickets, microcytic anemia, and neurotoxicity)
in aluminum containing antacids [27] and
sucralfate, and risk of milk alkali syndrome (hypercalcemia,
alkalosis, and renal failure [28] with calcium
containing antacids.
Histamine-2 receptor
antagonists (H 2RA):
like ranitidine or famotidine are short acting
(6 hours) acid suppressants but have rapid onset
of action (in 30 minutes) and can be used for
on-demand therapy (SOS therapy) but they develop
tachyphylaxis on long-term use (in 6 weeks)
[29]. Hence, they cannot be used for long term
therapy. Other problem with H2RAs
is a lack of post-prandial acid suppressant
effect. Overall, H2RAs
are less effective than PPI.
Proton pump inhibitors (PPIs):
They are also called Na-K-ATPase inhibitors as
they inhibit acid secretion by irreversibly
blocking this enzyme in the apical membrane of
parietal cells. PPIs should be protected from
gastric acid (gets inactivated in acidic media)
and that is why preparations are either enteric
coated microspheres (mouth dissolving tablets)
or capsules. Since they act best in activated
parietal cells, PPIs should be taken 30 minutes
before breakfast as parietal cells get activated
in response to a meal. Once daily dosing is
adequate and children (< 10 years) often require
a higher per kilogram dose (2-2.5mg/kg/day for
omeprazole and 1.4 mg/kg/day for lansoprazole)
than adults to obtain a similar degree of acid
suppression due to higher metabolism of the drug
[23,30,31]. The advantages of PPIs are; more
effective in relieving symptoms and healing
esophagitis than any other acid suppressants,
prolonged action (requires once daily dose), no
tachyphylaxis on prolonged use, and relatively
safe drug on long term use. Furthermore, due to
their strong acid suppression ability, PPIs
decrease 24-hour gastric secretion volumes and
thereby facilitate gastric emptying [30]. As it
takes 2 to 8 days for them to have maximum
effect, there is no role of PPIs in on-demand
therapy [32,33]. Of the various PPIs (omeprazole,
lansoprazole, esomeprazole, rabeprazole,
pantoprazole) there is no difference in efficacy
of one over the other. Out of all omeprazole,
lansoprazole and esomeprazole are FDA approved
for pediatric use.
Side effects of different
PPIs are almost similar and mild side effects
have been reported in up to 14% of children. The
most common side effects are headache, diarrhea,
constipation and nausea [34,35]. PPIs have been
safely used in children for up to 11 years [36].
Prokinetics: There is
insufficient evidence to justify the routine use
of prokinetics (metoclorpropamide, domperidon or
itopride) in the management of GERD [14]. The
only situation where prokinetics may be of some
use is GERD with associated gastroparesis.
Duration of medical therapy
GERD needs profound acid
suppression for a longer duration of time. PPI
therapy is recommended for at least 12 weeks and
then to taper over 2 to 3 months as rebound
hyperacidity is known after sudden stoppage of
PPI [37]. In a diagnosed case of GERD, if there
is no symptomatic improvement in 4 weeks then
the dose of PPI needs to be increased. If there
is a relapse on withdrawal of PPI, medication
needs to be restarted. Frequent relapses or
continuous symptoms are indications for
prolonged PPI therapy or surgery. In erosive
esophagitis, repeat endoscopy to document
healing is indicated at the end of 12 weeks
course of PPI therapy, as the risk of relapse is
more in those who do not show mucosal healing
than those who do. In a long term follow-up
study in children, it has been shown that
prolonged PPI therapy (median 3 years and up to
12 years) is safe. Regarding the dose of PPI in
maintenance therapy, it has been shown that full
healing dose is superior to half dose
therapy[38].
Surgery
Nissen fundoplication (open
or laparoscopic) may be of benefit in children
with confirmed GERD who have failed optimal
medical therapy, or who are dependent on medical
therapy for a long time, or who are
significantly noncompliant to medical therapy,
or who have life-threatening complications of
GERD. The point to be remember here is that
children who need surgery most (neurologically
impaired), develop surgery related complications
and surgical failure most. Almost two thirds of
neurologically impaired children and one thirds
of otherwise healthy children develop surgical
failure and require long-term medical treatment
[1]. Fundoplication in early infancy has a
higher failure rate than in late childhood [1,
14].
Bronchial asthma and GERD
The clinical association of
bronchial asthma and GERD is very strong but
causal relationship between these two entities
has not yet been established. Around 30% to 50%
of children with persistent, severe asthma have
GERD symptoms like heartburn but there is no
clinical association of mild, intermittent
asthma and GERD [39, 40]. It is not yet clear
which one causes what. Is it asthma that causes
GERD or is it GERD that causes asthma?
Pathophysiologically, either is possible. In
asthma, severe cough increases intra-abdominal
pressure and decreases intra-thoracic pressure
thereby changes the pressure gradient between
stomach and esophagus, hyper-inflated lungs
alter the relation between crural diaphragm and
gastro-esophageal junction, some asthma
medicines like beta-agonist decreases LES
pressure. All these factors predispose a child
with bronchial asthma to reflux. On the other
hand, reflux of gastric content can cause
bronchospasm by reflux or reflex mechanism.
Irritation of esophagus by acid reflux can
initiate reflex bronchospasm (reflex theory) as
both airway and esophagus share common autonomic
nerve supply. Other mechanism is
micro-aspiration (reflux theory) of gastric
contents which can trigger airway
hyper-responsiveness [39].
• Persistent asthma
with symptomatic GERD: can be
treated with PPI with a clear explanation
given to the patient and/or parents that
reflux symptoms will improve but chances of
improvement of asthma is remote.
• Intermittent asthma:
there is no clinical relation with GERD
• Difficult to control
asthma: (chronic symptoms, episodic
exacerbation, and continued requirement of
beta agonist despite inhaled
corticosteroids) or nocturnal asthma
symptoms: may derive some benefit from
long-term medical or surgical antireflux
therapy. It is recommended to perform pH
study before considering a trial of
long-term PPI therapy (14). However, recent
studies have refuted this recommendation.
Although some uncontrolled trials have shown
improvement in asthma with GERD treatment
[41] but a randomized placebo control trial
of omeprazole versus placebo in asthma
failed to show any benefit [42].
In a recent multicenter,
randomized, placebo-controlled trial from USA in
306 children, it was shown that lansoprazole, in
children with poor asthma control who were on
inhaled corticosteroid treatment, improved
neither symptoms nor lung function but was
associated with increased infection [40]. Hence,
PPI therapy for poorly controlled asthma without
overt GERD is not warranted.
GERD in neurologically
impaired children
Prevalence of GERD in
neurologically impaired children is much higher
than in neurologically normal children and the
prevalence is almost 50%. Severity and
complications of GERD is also much more in this
subset of patients. It has been shown that the
prevalence of erosive esophagitis is 30% to 70%
compared to just 5% in children without
neurological defects. This group of children
needs prolonged medication and more often
surgery [43].
Conclusions
GER is common in infants but
GERD is not so common in early childhood. Most
infants have physiological reflux and need
minimal intervention as their symptoms resolve
by 18 months of age. There is no gold standard
diagnostic test for GERD and investigations
should be tailored to the clinical concern for a
given child. Empirical PPI therapy for 4 weeks
is justified in older children and adolescents
with classical symptoms. For extraesophageal
manifestations, pH-metry with or without
impedance and for esophagitis, endoscopy is the
best investigations. Medical therapy with PPI is
very effective and safe. Surgical therapy is not
a panacea as it carries significant morbidity
and often fails in those who need it most.
Funding: None;
Competing interests: None stated.
References
1. Hassall E. Outcomes of
fundoplication: causes for concern,newer
options. Arch Dis Child. 2005;90:1047-52.
2. Nelson SP, Chen EH, Syniar
GM, Christoffel KK. Prevalence of symptomatic
gastroesophageal reflux during infancy. A
pediatric practice-based survey, pediatric
practice research group. Arch Pediatr Adolesc
Med. 1997;151:569-72.
3. Martin AJ, Pratt N,
Kennedy D, Ryan P, Ruffin RE, Miles H, et al.
Natural history and familial relationships of
infant spilling to 9 years of age. Pediatrics.
2002;109:1061-7.
4. Campanozzi A, Bossia G,
Pensabene L, Panetta F, Marseglia A,
Strisciuglio P, et al. Prevalence and
natural history of gastroesophageal reflux:
pediatric prospective survey. Pediatrics.
2009;123:779-83.
5. De S, Rajeshwari K, Kalra
KK, Gondal R, Malhotra V, Mittal SK.
Gastroesophageal reflux in infants and children
in north India. Trop Gastroenterol.
2001;22:99-102.
6. Carroll MW, Jacobson K.
Gastroesophageal reflux disease in children and
adolescents: when and how to treat. Pediatr
Drugs. 2012;14:79-89.
7. Nelson P, Chen EH, Syniar
GM, Christoffel KK. Prevalence of symptomatic
gastroesophageal reflux during childhood: A
pediatric practice-based survey, pediatric
practice research group. Arch Pediatr Adolesc
Med. 2000;154:150-4.
8. Michail S.
Gastroesophageal reflux. Pediatr Review.
2007;28:101-3.
9. Tolia V, Vandenplas Y.
Systematic review: the extra-esophageal symptoms
of gastroesophageal reflux disease in children.
Aliment Pharmacol Ther. 2009;29:258-72.
10. Orenstein SR, Shalaby TM,
Cohn JF. Reflux symptoms in 100 normal infants:
diagnostic validity of the infant
gastroesophageal reflux questionnaire. Clin
Pediatr. 1996;35:607-14.
11. Hyman PE, Milla PJ,
Benninga MA, Davidson GP, Fleisher DF, Taminiau
J. Childhood functional gastrointestinal
disorders: Neonate/Toddler. Gastroenterology.
2006;130:1519-26.
12. Aggarwal S, Mittal SK,
Kalra KK, Rajeshwari K, Gondal R. Infant
gastroesophageal reflux disease score:
reproducibility and validity in a developing
country. Trop Gastroenterol. 2004;25:96-8.
13. Talley NJ, Armstrong D,
Junghard O, Wiklund I. Predictors of treatment
response in patients with non-erosive reflux
disease. Aliment Pharmacol Ther. 2006;24:371-6.
14. Vandenplas Y, Rudolph CD,
Di Lornzo C, Hassall E, Liptak G, Muzur L, et
al. Pediatric Gastroesophageal reflux
clinical practice guidelines: joint
recommendations of the North American Society
for Pediatric Gastroenterology, Hepatology and
Nutrition (NASPGHAN) and the European Society
for Pediatric Gastroenterology, Hepatology and
Nutrition (ESPGHAN). J Pediatr Gastroenterol
Nutr. 2009;49:498-547.
15. Wenzl TG. Role of
diagnostic tests in GERD. J Pediatr
Gastroenterol Nutr. 2011; 53 (Suppl 2): S4-6.
16. Wenzl TG, Benninga MA,
Loots CM, Salvatore S, Vandenplas Y, on behalf
of the ESPGHAN EURO-PIG working group.
Indications, methodology and interpretation of
combined esophageal impedance-pH monitoring in
children: ESPGHAN EURO-PIG standard protocol. J
Pediatr Gastroenterol Nutr. 2012;55:230-4.
17. Vieira MC, Pisani JC,
Mulinari RA. Diagnosis of reflux esophagitis in
infants: histology of the distal esophagus must
complement upper gastrointestinal endoscopy. J
Pediatr. (Rio J) 2004;80:197-202.
18. Boccia G, Manguso F,
Miele E, Buonavolonta R, Staiano A. Maintenance
therapy of erosive esophagitis in children after
healing by omeprazole: is it advisable? Am J
Gastroenterol. 2007;102:1291-7.
19. Rudolph CD, Mazur LJ,
Liptak GS, Baker RD, Boyle JT, Colletti RB,
et al. Guidelines for evaluation and
treatment of gastroesophageal reflux in infants
and children: Recommendations of the North
American Society for Pediatric Gastroenterology
and Nutrition. J Pediatr Gastroenterol Nutr.
2001;32:S1-31.
20. Thompson JK, Koehler RE,
Richter JE. Detection of gastroesophageal
reflux: value of barium studies compared with
24-hr pH monitoring. Am J Roentgenol.
1994;162:621-6.
21. Chen MY, Ott DJ, Sinclair
JW, Wu WC, Gelfand DW. Gastroesophageal reflux
disease: correlation of esophageal pH testing
and radiographic findings. Radiology.
1992;185:483-6.
22. Horvath A, Dziechciarz P,
Szajewska H. The effect of thickened-feed
interventions on gastroesophageal reflux in
infants: systematic review and meta-analysis of
randomized, controlled trials. Pediatrics.
2008;122:e1268-77.
23. Cezard JP. Managing
gastro-esophageal reflux disease in children.
Digestion. 2004;69:3-8.
24. Orenstein SR, Hassall E,
Furmaga-Jablonska W, Atkinson S, Raanan M.
Multicenter, double-blind, randomized,
placebo-controlled trial assessing efficacy and
safety of proton pump inhibitor lansoprazole in
infants with symptoms of gastroesophageal reflux
disease. J Pediatr. 2009;154:514-20.
25. Hassall E, Israel D,
Shepherd R, Radke M, Dalvag A, Skold B, et al.
Omeprazole for treatment of chronic erosive
esophagitis in children: a multicenter study of
efficacy, safety, tolerability and dose
requirements, International Pediatric Omeprazole
Study Group. J Pediatr. 2000;137:800-7.
26. Tolia V, Ferry G,
Gunasekaran T, Huang B, Keith R, Book L.
Efficacy of lansoprazole in the treatment of
gastroesophageal reflux disease in children. J
Pediatr Gastroenterol Nutr. 2002;35:S308-S318.
27. Sedman A. Aluminum
toxicity in childhood. Pediatr Nephrol.
1992;6:383-93.
28. Beall DP, Henslee HB,
Webb HR, Scofield RH. Milk-alkali syndrome: a
historical review and the description of the
modern version of the syndrome. Am J Med Sci.
2006; 331:233-42.
29. Hyman PE, Garvey TQ 3rd,
Abrams CE. Tolerance to intravenous ranitidine.
J Pediatr. 1987;110:794-6.
30. Israel DM, Hassall E.
Omeprazole and other proton pump inhibitors:
pharmacology, efficacy, and safety, with special
reference to use in children. J Pediatr
Gastroenterol Nutr. 1998;27:568-79.
31. Litalien C, Theoret Y,
Faure C. Pharmacokinetics of proton pump
inhibitors in children. Clin Pharmacokinet.
2005;44:441-66.
32. Savarino V, di Mario F,
Scarpignato C. Proton pump inhibitors in GORD:
an overview of their pharmacology, efficacy and
safety. Pharmacol Res. 2009;59:135-53.
33. Kearns GL, Winter HS.
Proton pump inhibitors in pediatrics: relevant
pharmacokinetics and pharmacodynamics. J Pediatr
Gastroenterol Nutr. 2003;37:S52-9.
34. Zhao J, Li J,
Hamer-Maansson JE, Andersson T, Fulmer R,
Illueca M,. Pharmacokinetic properties of
esomeprazole on children aged 1 to 11 years with
symptoms of gastroesophageal reflux disease: a
randomized, open-label study. Clin Ther.
2006;28: 1868-76.
35. Li J, Zhao J,
Hamer-Maansson JE, Andersson T, Fulmer R,
Illueca M, et al. Pharmacokinetic
properties of esomeprazole in adolescent
patients aged 12 to 17 years with symptoms of
gastroesophageal reflux disease: a randomized,
open-label study. Clin Ther. 2006;28:419-27.
36. Hassall E, Kerr W, El-Serag
HB. Characteristics of children receiving proton
pump inhibitors continuously for up to 11 years
duration. J Pediatr. 2007;150:262-7, 267.e1
37. Fossmark R, Johnsen G,
Johanessen E, Waldum HL. Rebound acid hyper
secretion after long-term inhibition of gastric
acid secretion. Aliment Pharmacol Ther.
2005;21:149-54.
38. Illueca M, Wernersson B,
Henderson C, Lundborg P. Maintenance treatment
with proton pump inhibitors for reflux
esophagitis in pediatric patients: a systematic
literature analysis. J Pediatr Gastroenterol
Nutr. 2010;51:733-40.
39. Gold BD.Asthma and
gastroesophageal reflux disease in children:
exploring the relationship. J Pediatr.
2005;146: S13-S20.
40. Writing committee for the
American Lung Association Asthma Clinical
Research Centers, Holbrook JT, Wise RA, Gold BD,
Blake K, Brown ED, et al. Lansoprazole
for children with poorly controlled asthma.
JAMA. 2012;307:373-81.
41. Khoshoo V, Haydel R Jr.
Effect of antireflux treatment on asthma
exacerbation in nonatopic children. J Pediatr
Gastroenterol Nutr. 2007;44:331-5.
42. Stordal K, Johannesdottir
GB, Bentsen BS, Knudsen PK, Carlsen KC, Closs O,
et al. Acid suppression does not change
respiratory symptoms in children with asthma and
gastro-esophageal reflux disease. Arch Dis
Child. 2005;90:956-60.
43. Bohmer CJM,
Klinkenberg-Knol EC, Niezen-de Boer MC,
Meuwissen SGM. Gastroesophageal reflux disease
in intellectually disabled individuals: how
often, how serious, how manageable? Am J
Gastroenterol. 1999;94:804-10.
|
|
|
 |
|

