|
|
|
Indian Pediatr 2012;49: 35-41
|
 |
Clofibrate for Unconjugated Hyperbilirubinemia
in Neonates: A Systematic Review |
|
Tao Xiong, Dapeng Chen, Zhoujin Duan, Yi Qu and Dezhi Mu*
From the Department of Pediatrics, West China Second
University Hospital, Sichuan University, Chengdu, China and *Department of
Neurology and Pediatrics, Newborn Brain Research Institute, University of
California, San Francisco, California, USA.
Correspondence to: Dezhi Mu, Department of Pediatrics,
West China Second University Hospital,
Sichuan University, China.
Email:
[email protected]
|
|
Abstract
Objective: To evaluate the effect of clofibrate
for unconjugated hyperbilirubinemia in neonates.
Methods: A systematic review with meta-analysis
of randomized controlled trials or quasi-randomized controlled trials
was conducted to evaluate the clofibrate treatment in neonates with
unconjugated hyperbilirubinemia. We followed the guidelines from the
Cochrane review group and the PRISMA statement.
Results: Of 148 studies identified, a total of
13 studies on 867 infants were included. A single oral administration
of clofibrate was associated with decreased need of phototherapy
(RR:.38, 95% CI: 0.21 to 0.68), shortened duration of phototherapy
(mean duration: 23.88 h, 95% CI: 33.03 to -14.72 h) and reduced peak
total serum bilirubin (mean duration: -1.62 mg/dL, 95% CI: 2.13 to
-1.11 mg/dL). These effects were especially obvious in term infants
and infants without hemolytic diseases. Data regarding mortality or
kernicterus were not available from included studies.
Conclusions: Clofibrate may have short-term
benefits for the infants with hyperbilirubinaemia, especially for
population of term infants and infants without hemolytic diseases.
Large RCTs with long-term followup are required to verify the safety
of clofibrate and assess its long-term effects.
Key words: Clofibrate, Jaundice, Management, Meta-analysis,
Newborn, Phototherapy.
|
|
Neonatal jaundice is one of the most common
conditions confronting neonatologists. Epidemiologic studies show that
about 60% of term and 80% of preterm babies develop jaundice in the first
week of life [1]. The goal of the management of unconjugated
hyperbilirubinemia is to avoid bilirubin toxicity [2]. Exchange
transfusion and phototherapy are two leading treatments for severe
jaundice. Although the need for exchange transfusion has markedly
decreased after the availability of effective phototherapy, a small
proportion of infants with severe hyperbilirubinemia need exchange
transfusion, which leads to increased risk of infections and death [3,4].
Clofibrate, an activator of peroxisome receptors,
increases the hepatic conjugation of unconjugated bilirubin by inducing
activity of glucuronyl transferase [5,6]. In 1981, Lindenbaum, et al.
[7] published the first randomized controlled trial (RCT) for the use of
clofibrate in neonates with jaundice. Since then, a series of clinical
trails have reported that clofibrate could decrease the need of
phototherapy and exchange transfusion by decreasing the peak serum
bilirubin and duration of hyper-bilirubinemia. We therefore conducted this
systematic review and meta-analysis to evaluate the effect of clofibrate
in neonates with unconjugated hyperbilirubinemia.
Methods
Data sources
We followed the guidelines from the Cochrane review
group for undertaking and reporting this systematic review and
meta-analysis [8]. The published medical literature in the Medline, Embase,
Cochrane Central Register of Controlled Trials (CCTR) and ISI Web of
Knowledge (SCI) databases were searched in October, 2010. The reference
lists of identified studies and key review articles were also searched.
Abstracts of the National and International American Pediatric
Society/Pediatric Academic Societies, The European Paediatric Research
Societies and the Effective Care of the Newborn Infant were hand searched
for unpublished articles (up to 2010). No language restriction was
applied. Two authors independently searched these databases by using the
subject headings terms "clofibrate", "hyperbiliru-binemia", "hyperbiliru-binemia,
neonatal", "jaundice", "jaundice, neonates" and the key words "clofibrate",
"jaundice", "hyperbiliru-binemia". Studies with titles or abstracts that
discussed clofibrate for jaundice were retrieved.
Study selection
Inclusion criteria for trials included (i) age
<28 days; (ii) unconjugated hyperbilirubinemia (irrespective of
etiology and defined as conjugated bilirubin less than 2 mg/dL); (iii)
clofibrate administration for prevention or treatment of unconjugated
hyperbilirubinemia; (iv) RCT or quasi- RCT (parallel group
/crossover); (v) trials with at least one of the outcome parameters
in this review (see below). All articles were initially screened by title,
abstract, and keywords. When appropriateness of the article could not be
determined, the full article was obtained. Two authors independently
screened the studies for eligibility. Any disagreement was resolved
through discussion to reach a consensus.
Data extraction
The following data were extracted and put into the
standardized forms: author, publication year, characteristics of neonates
(gestational age, birth weight, causes of jaundice, postnatal age and
level of total serum bilirubin (TSB) at admission), dose of clofibrate,
criteria for phototherapy and exchange transfusion, and follow-up periods.
Outcomes included the need of phototherapy (for the trials which started
phototherapy on admission, the need of phototherapy was assessed at 48-72
h after clofibrate administration and for prophylactic administration, it
was assessed at the end of study), the need of exchange transfusion,
duration of phototherapy, peak TSB (the highest TSB level after clofibrate
administration), morbidity of kernicterus, and side effects of treatment
(vomiting, loose stools, leucopenia, renal failure, abnormal liver
function tests, etc).
Quality assessment of studies
The quality of the studies was assessed according to
the standardized criteria of the Cochrane Database of Systematic Reviews.
The methodological quality of each trial was assessed independently by two
authors. For each trial, information was sought regarding the method of
randomization, allocation concealment, blinding of intervention, blinding
of outcome assessment and reporting of the complete outcome. The unstated
details were acquired through communication with the authors of the
trials.
Statistical analysis
Meta-analysis of the included trials was performed
using RevMan 5. For categorical outcomes, the relative risk (RR), the risk
difference (RD) and 95% confidence intervals (CIs) were calculated. For
continuous outcomes, mean difference (MD) and 95% CIs were calculated.
Heterogeneity was measured by using the I 2
test [9]. Data without heterogeneity (I2
<50%) were combined by fixed-effects model [10]. When there
was unexplained heterogeneity, we incorporated it into a random-effects
model [11]. Subgroup analyses were conducted according to causes of
jaundice (with/without hemolytic diseases) and term/preterm status of
neonates. Potential publication bias was assessed by funnel plot [12]. A
P value of <0.05 was considered statistically significant.
Results
Studies and participants
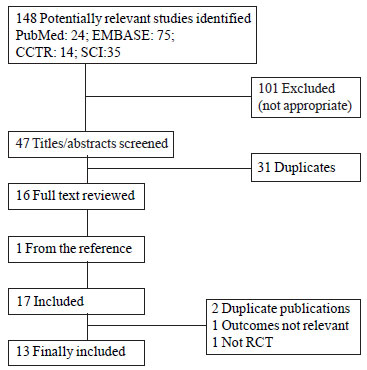
148 articles were retrieved on the basis of the general
search strategy. Of them, two authors reached a complete consensus that 13
RCTs with 867 neonates met the inclusion criteria and were selected for
analyses (Fig. 1). The trial dates ranged from 1981 to 2010;
two of 13 trials were published in French [7, 13], one in Spanish [14] and
ten in English [15-24].
 |
|
Fig. 1 Results of search strategy of
systematic review. |
Table I presents the characteristic of subjects
in included trials. Eight of the 13 trials included only term infants,
four trials only preterm infants, and one trial included both. The average
birth weight ranged from 1879 g to 3370 g. The average TSB levels at
admission were from 5.9 to 23.1 mg/dL.
TABLE I Characteristics of Subjects in Trials
|
Author, |
N clofibrate/ |
Gestational |
Birth |
TSB levels at |
Hemolytic |
|
Year [Ref.] |
control |
age |
Weight
(g) |
admission (mg/dL) |
disease |
|
Lindenbaum,1981 [7] |
9347/46 |
Term |
3370±105 |
14.3±0.4 |
22 with ABO
incompatibility |
|
Lindenbaum,1985 [13] |
8946/43 |
Preterm (31-36) |
1879±221 |
unclear |
Without |
| Flores
Nava,1996 [14] |
4522/23 |
Preterm/ term |
2754±803 |
unclear |
Included ABO and |
| |
|
(34 -42) |
|
|
Rh
incompatibility* |
|
Mohammadzadeh, 2005 [15] |
6030/30 |
Term |
3260±481 |
23.1±3.4 |
Without |
| Moslehi,
2007 [16] |
9060/30 |
Term |
2543±548 |
17.6±1.4 |
Without |
| Eghbalian,
2007 [17] |
6030/30 |
Term |
>2500 |
20.9±3.6 |
Without
|
|
Zahedpasha,2007 [18] |
6030/30 |
Term |
3133±456 |
17.9±2.1 |
Without |
| Badeli,
2008 [19] |
9045/45 |
Term |
3171±278 |
18.4±1.6 |
Without |
|
Mohammadzadeh, 2008 [20] |
5226/26 |
Preterm (31.5±1.5) |
1369±201 |
5.9±2.4 |
Without |
|
Zahedpasha, 2008 [21] |
4021/19 |
Term |
3258±479 |
18.0±1.9 |
G6PD
deficient |
|
Mohammadzadeh, 2009 [22] |
6030/30 |
Preterm (31.5±1.5) |
2114±328 |
21.1±5.2 |
Without |
| Sakha,
2009 [23] |
6835/33 |
Preterm (34 -37) |
2359±535 |
19.8±2.4 |
Without |
| Sharafi,
2010 [24] |
6030/30 |
Term |
3129±431 |
17.3±1.5 |
Without |
|
*11 cases with ABO
incompatibility, 1with Rh incompatibility; TSB: total serum bilirubin. |
Intervention
The average age at admission varied from 2 to 9.2 days.
Neonates in all trials received a single oral dose of clofibrate within
the first 14 days after birth. Clofibrate was dissolved in solution (corn
oil or water), and was given orally with/without orogastric tubes. The
dose of clofibrate ranged from 25 mg/kg to 100mg/kg. Phototherapy was
given on admission [15-19, 21-24] or when TSB was over certain threshold
respectively [7,14,20]. Exchange transfusion was given when TSB was not
well controlled by clofibrate and phototherapy in four trials [7, 13-14,
22]. The main characteristics of these interventions are described in
Table II.
TABLE II Characteristics of Interventions in Trials
Author,
Year [Ref] |
Age at
admission |
clofibrate
dose* |
Threshold of phototherapy
(mg/dL TSB) |
Threshold of
exchange |
Follow-up for
side effects |
| |
(days) |
(mg/ kg) |
|
|
transfusion |
(after discharge) |
| |
|
|
Start |
end |
|
|
|
Lindenbaum,1981 [7] |
2-3 |
50 |
>17.5 |
unclear |
unclear |
Without |
|
Lindenbaum,1985 [13] |
2-3 |
100 |
Unclear |
unclear |
unclear |
12 days |
|
Flores Nava, 1996 [14] |
< 1.5 |
100 |
Indirect |
Indirect |
Jasso’s |
unclear |
| |
|
|
bilirubin† |
bilirubin‡ |
Standard |
|
|
Mohammadzadeh, 2005 [15] |
9±4 |
100 |
on admission |
<14mg/dL |
>30 or 25 mg/dL |
2 days |
|
Moslehi, 2007 [16] |
5.2±1.9 |
50 /25 |
on admission |
unclear |
unclear |
2 days |
|
Eghbalian, 2007 [17] |
Most 2-3 |
100 |
on admission |
<12mg/dL |
>30 or 25 mg/dL |
1 week |
|
Zahedpasha, 2007 [18] |
6.0±2.9 |
100 |
on admission |
<10mg/dL |
TSB >25 mg/dL |
1 week |
|
Badeli, 2008 [19] |
5.3±1.8 |
100 |
on admission |
unclear |
unclear |
1 month |
|
Mohammadzadeh, 2008 [20] |
unclear |
100 |
5 or 7mg/dL§
|
≤50% of photo-
therapy level |
unclear |
unclear |
|
Zahedpasha, 2008 [21] |
5.1±2.3 |
100 |
on admission |
< 10mg/dL |
unclear |
1 week |
|
Mohammadzadeh, 2009 [22] |
9.2±5.4 |
100 |
on admission |
unclear |
unclear |
1 week |
|
Sakha, 2009 [23] |
6.1±2.9 |
100 |
on admission |
2004 AAP
guidelines|| |
unclear |
1 week |
|
Sharafi, 2010 [24] |
6.7±2.9 |
50 |
on admission |
<10mg/dL |
unclear |
2 months |
*All studied used a single oral dose; † Indirect bilirubin >4 mg/dL in umbilical cord blood,
>6 mg/dL within 12 h of life; >10 mg/ dL within 24 h, >13 mg/dL within 48 h,
and >15 mg/dL at any time; ‡Indirect bilirubin <10mg/dL or <admission level-2mg/dL;
§Reach to 5mg/dL in birth weight in birth weight less than 1,000 g, 7mg/dL in birth weight 1,000~1,500 g;
|| American Academy of Pediatrics Subcommittee on Hyperbilirubinemia.
|
Methodologic quality
For most of the studies, both evaluators reached a high
degree of agreement for study-quality assessment. Disagreements existed in
three studies where there were no details regarding allocation concealment
and blinding [15,20,22]. However, these disagreements were resolved after
contacting the authors. All were randomized/quasi-randomized trials,
although the methods of randomization in two studies were not clearly
stated [18,21]. In six trials, randomized allocation was concealed from
the physicians [7,13,15,20,22-23]. In six studies, intervention was
blinded to the physicians, nurses and parents by use of placebos, and
blinded outcome assessments were concealed from physicians and clinical
technologists [7, 13,15,20,22-23]. One trial had incomplete outcome
reporting [14]. Other twelve trials had complete reporting of in-hospital
outcomes for infants, without possible attrition bias through withdrawals
and dropouts [Web Table I].
The funnel plot for the primary outcome of peak TSB did
not show any publication bias in this review (Web Fig. 1).
Outcomes
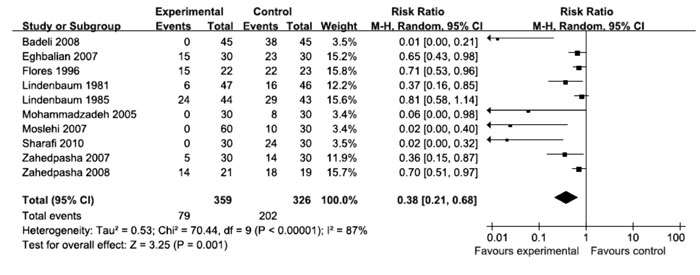
Phototherapy: Need for phototherapy was
significantly reduced in the clofibrate treated infants in meta-ten trials
(Fig. 2). Subgroup analysis showed that the reduction in RR for the
need of phototherapy was prominent in infants without hemolytic diseases
rather than with hemolytic diseases, and in term infants rather than in
preterm infants.
 |
|
Fig. 2 Need of phototherapy. |
 |
|
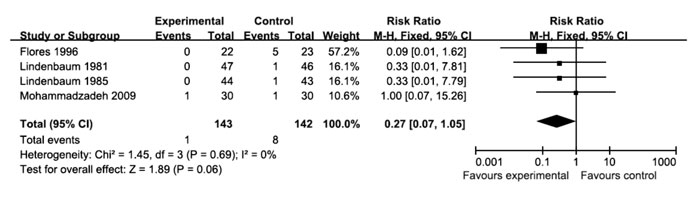
Fig. 3 Need of exchange transfusion |
Duration of phototherapy was reported in 7 out of the
13 trials. Clofibrate treatment resulted in a shorter duration of
phototherapy than that in control group (Fig. 4). The
subgroup meta-analysis revealed a significant decrease in the duration of
phototherapy for infants without hemolytic disease, and for term or
preterm infants. There was no information about phototherapy duration in
infants with hemolytic disease (Table III).
TABLE III Meta-analyses of Outcomes
|
Outcome |
No of studies |
No of cases |
Measure (95% CI)† |
|
Need of phototherapy |
|
All infants |
10 |
685 |
RR: 0.38 [0.21, 0.68]; RD: -0.38 [-0.57, -0.18] |
|
infants without HD* |
8 |
578 |
RR: 0.17 [0.06, 0.48]; RD: -0.42 [-0.64, -0.19] |
|
infants with HD |
2 |
62 |
RR: 1.00 [0.35, 2.86]; RD: -0.06 [-0.56, 0.44] |
|
term infants |
8 |
553 |
RR: 0.20 [0.07, 0.54]; RD: -0.42 [-0.64, -0.20] |
|
preterm infants |
1 |
87 |
RR: 0.81 [0.58, 1.14]; RD: -0.13 [-0.33, 0.07] |
|
Need of exchange transfusion |
|
All infants |
4 |
285 |
RR: 0.27 [0.07, 1.05]‡; RD: -0.05 [-0.09,-0.00]‡ |
|
infants without HD |
2 |
147 |
RR: 0.59 [0.08, 4.37]‡; RD: -0.01 [-0.07, 0.04]‡ |
|
infants with HD |
1 |
22 |
RR:0.47 [0.02, 10.32]‡; RD: -0.08 [-0.29, 0.13]‡ |
|
term infants |
1 |
93 |
RR:0.33 [0.01, 7.81]‡; RD: -0.02 [-0.08, 0.04]‡ |
|
preterm infants |
2 |
147 |
RR: 0.59 [0.08, 4.37]‡; RD: -0.01 [-0.07, 0.04]‡ |
|
Duration of phototherapy (hs) |
|
All infants |
7 |
465 |
MD: -23.88 [-33.03, -14.72] |
|
infants without HD* |
6 |
420 |
MD: -21.50 [-30.68, -12.32] |
|
infants with HD |
0 |
0 |
|
|
term infants |
4 |
300 |
MD: -19.95 [-31.22, -8.67] |
|
preterm infants |
2 |
120 |
MD: -25.00 [-33.75, -16.25]‡ |
|
Peak TSB (mg/dL) |
|
All infants |
12 |
790 |
MD: -1.62 [-2.13, -1.11] |
|
infants without HD |
11 |
728 |
MD: -1.69 [-2.17, -1.21] |
|
infants with HD |
2 |
62 |
MD: -0.48 [-2.04, 1.08] |
|
term infants |
8 |
553 |
MD: -1.89 [-2.56, -1.22] |
|
preterm infants |
4 |
237 |
MD: -0.97 [-2.23, 0.28] |
|
* HD represents hemolytic disease; † Most of following
outcome using randomized-effects model because of statistical
heterogeneity; ‡ Given without statistical heterogeneity
fix-effects model used. |
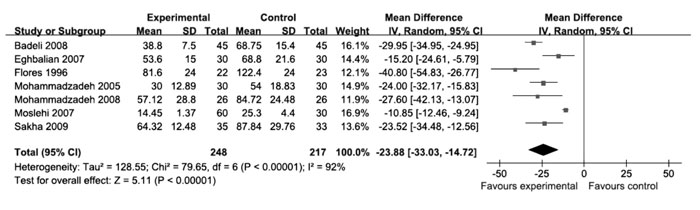 |
|
Fig. 4 Duration of phototherapy. |
Need of exchange transfusion: Meta-analysis
of four trials reporting the need of exchange transfusion did not reveal a
statistically significant difference in need for exchange transfusion (Fig.
3). Clofibrate treatment did not significantly decrease the need of
exchange transfusion in infants with or without hemolytic disease, and in
term infants or preterm infants.
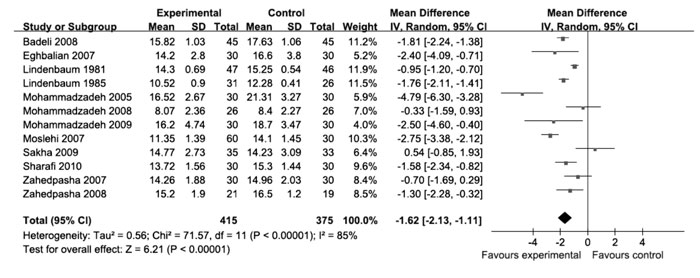
Peak TSB concentration: Peak TSB was significantly
lower in clofibrate group (Fig. 5). Significantly reduced
TSB levels were seen in infants without hemolytic diseases and in term
infants. In infants with hemolytic diseases and in preterm infants, effect
was not significant. Mortality and Kernicterus: No study reported
outcome of death or Kernicterus.
 |
|
Fig. 5 Peak TSB. |
Side effects: In the included studies, side effects
were assessed through clinical observation and laboratory tests [complete
blood count, total serum bilirubin, blood urea nitrogen, blood creatinine,
liver function tests (SGOT, SGPT)] during the follow-up periods ranging
between 2 days to 2 months. Only one infant had cholestasis with direct
bilirubin 3.9 mg/dL after three days of clofibrate. No other side effects
were reported.
Discussion
In this systematic review, we found that clofibrate-treatment
reduced the need of phototherapy, shortened duration of phototherapy, and
resulted in a lower peak TSB. These beneficial effects were prominent in
subgroups of infants without hemolytic diseases and in term infants.
Clofibrate treatment did not show prominent effects in infants with
hemolytic diseases. However, the number of infants with hemolytic diseases
included in the review was too small to draw any meaningful conclusion.
The absence of significant therapeutic effect of
clofibrate in preterm infants could be because of insufficient sample
size, and also due to its different metabolism in preterm infants. The
main metabolite of clofibrate is clofibric acid, which has the effective
plasma concentration of 140µg/mL for jaundiced neonates [13]. In humans,
most of the plasma clofibric acid is bound to albumin. Thus, decreased
level of albumin in preterm infants could lead to increased free form of
clofibric acid, which facilitates the clearance of clofibric acid and
results in lower plasma levels of clofibric acid. Because preterm infants
have lower level of albumin, the dose of clofibrate needs to be adjusted
according to the gestational age: 100 mg / kg for 34 to 36 weeks of
gestational age, and >100 mg/kg for 31 to 33 weeks [13]. Lower dose of clofibrate for preterm infants in some studies may explain the lack of a
significant effect.
Short-term safety of clofibrate treatment was good in
the included studies, except for a single case of transient cholestasis.
Clofibrate has been found to be carcinogenic in rodents but
epidemiological and observational studies have not found any such evidence
in adult humans [25-27]. It is not known whether long-term carcinogenesis
could occur in neonates with clofibrate treatment. However, in most of the
studies, tests for liver or muscle enzymes were not done, and the
follow-up periods were too short (<2 month).
The methodological quality varied among studies. In
some trials, allocation concealment was unclear or inappropriate which
might have resulted in overestimation of the intervention effect. Due to
the lack of blinding of intervention in several trials, treatment bias
could have occurred. Moreover, sample sizes in included trials were
generally small.
A major limitation of this meta-analysis is the
statistical heterogeneity. Although subgroup analyses (cause of jaundice,
gestational age) and the sensitivity analyses (dose of clofibrate,
publication year or location) were done, the heterogeneity remains
unsolved. This heterogeneity resulted from the difference of baseline TSB
at admission, the varied causes of jaundice, the thresholds for
phototherapy and exchange transfusion, the different methods of TSB
measurement, and the genetic factors between different nations.
This meta-analysis shows that clofibrate may have
short-term benefits for the infants with hyper-bilirubinemia, especially
in term infants and infants without hemolytic diseases. At present, there
is no evidence to show whether clofibrate treatment modifies the risk of
death, kernicterus or long-term neurodevelop-mental impairment due to
bilirubin encephalopathy.
Long-term developmental follow-up is required to assess
the safety of clofibrate treatment, confirm its long-term benefits in
different settings, and address its optimal therapeutic dose in preterm
neonates and infants with hemolytic diseases.
Contributors: TX: writing the review; DC: data
collection and writing the draft; ZD: responsible for data collection and
study-quality assessment; YQ: provide support for the analysis of the
data; and, DM: designing the review and supporting the publication.
Funding: National Natural Science Foundation of
China (No. 31171020, No. 30825039 and No. 30973236). Ministry of Education
of China (No.IRT0935, No.20070610092). Science and Technology Department
of Sichuan Province (No.2010SZ0280).
Competing interests: None stated.
References
1. Rennie J, Burman-Roy S, Murphy MS. Neonatal
jaundice: summary of NICE guidance. BMJ. 2010;340:c2409.
2. Suresh GK, Martin CL, Soll RF. Metalloporphyrins for
treatment of unconjugated hyperbilirubinemia in neonates. Cochrane
Database Syst Rev. 2003:CD004207.
3. Steiner LA, Bizzarro MJ, Ehrenkranz RA, Gallagher
PG. A decline in the frequency of neonatal exchange transfusions and its
effect on exchange-related morbidity and mortality. Pediatrics.
2007;120:27-32.
4. Alcock GS, Liley H. Immunoglobulin infusion for
isoimmune haemolytic jaundice in neonates. Cochrane Database Syst Rev.
2002:CD003313.
5. Cuperus FJ, Hafkamp AM, Hulzebos CV, Verkade HJ.
Pharmacological therapies for unconjugated hyperbilirubinemia. Curr Pharm
Dis. 2009;15:2927-38.
6. Wang G, Shen H, Rajaraman G, Roberts MS, Gong Y,
Jiang P, et al. Expression and antioxidant function of liver fatty
acid binding protein in normal and bile-duct ligated rats. Eur J Pharmacol.
2007;560:61-8.
7. Lindenbaum A, Hernandorena X, Vial M, Benattar C,
Janaud JC, Dehan M, et al. Clofibrate for the treatment of
hyperbilirubinemia in neonates born at term: a double blind controlled
study (author’s transl). Arch Fr Pediatr. 1981;38:867-73. (Article in
French).
8. Cochrane Neonatal Review Group. Guidelines for
Reviewers and Editors Available from: http://neonatalcochraneorg. Accessed
October 1, 2010.
9. Higgins JP, Thompson SG, Deeks JJ, Altman DG.
Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-60.
10. Mantel N, Haenszel W. Statistical aspects of the
analysis of data from retrospective studies of disease. J Natl Cancer
Inst. 1959;22:719-48.
11. DerSimonian R, Laird N. Meta-analysis in clinical
trials. Control Clin Trials. 1986;7:177-88.
12. Egger M, Davey Smith G, Schneider M, Minder C. Bias
in meta-analysis detected by a simple, graphical test. BMJ.
1997;315:629-34.
13. Lindenbaum A, Delaporte B, Benattar C, Dehan M,
Magny JF, Gerbet D, et al. Preventive treatment of jaundice in
premature newborn infants with clofibrate. Double-blind controlled
therapeutic trial. Arch Fr Pediatr. 1985;42:759-63. (Article in French).
14. Flores Nava G, Vargas Perez C, Lopez Padilla M,
Escobedo Chavez E. Clofibrate in the prevention of neonatal
hyperbilirubinemia. Practica Pediatrica. 1996;5:40-6.
15. Mohammadzadeh A, Farhat A, Iranpour R. Effect of
clofibrate in jaundiced term newborns. Indian J Pediatr. 2005;72:123-6.
16. Moslehi MA, Pishva N. Determination of effect of
low dose vs moderate dose clofibrate on decreasing serum bilirubin in
healthy term neonates. Iranian Journal of Pediatrics. 2007;17:108-12.
17. Eghbalian F, Pourhossein A, Zandevakili H. Effect
of clofibrate in non-hemolytic indirect hyperbiliru-binemia in full term
neonates. Indian J Pediatr. 2007;74:1003-6.
18. Zahedpasha Y, Ahmadpour-Kacho M, Hajiahmadi M,
Naderi S. Effect of clofibrate in jaundiced full-term infants:a randomized
clinical trial. Arch Iran Med. 2007;10:349-53.
19. Badeli H, Sharafi R, Sajedi S. The effect of
clofibrate on neonatal hyperbilirubinemia in uncomplicated jaundice.
Iranian Journal of Pediatrics. 2008;18:20-4.
20. Mohammadzadeh A, Farhat A, Jafarzadeh M,
Mirzarahimi M, Esmaili H, Amiri R. Prophylactic effect of clofibrate in
low birth weight neonates, hyperbilirubinemia. J Chinese Clinical Med.
2008;3:140-4.
21. Zahedpasha Y, Ahmadpour-Kacho M, Hajiahmadi M,
Naderi S, Kamali AA. Efficacy of clofibrate on severe neonatal jaundice
associated with glucose-6-phosphate dehydrogenase deficiency (a randomized
clinical trial). Southeast Asian J Trop Med Public Health. 2008;39:
557-61.
22. Mohammadzadeh A, Farhat AS, Amiri R, Esmaely H,
Bagheri S. Treatment effect of clofibrate in jaundiced low birth weight
neonates. International J Hematol Oncol. 2009;19:100-5.
23. Sakha SH, Gharehbaghi MM, Rahbani ME. The effect of
clofibrate with phototherapy in late pre-term newborns with non-hemolytic
jaundice. Indian J Med Sci. 2009;63:174-9.
24. Sharafi R, Mortazavi Z, Sharafi S, Parashkouh R.
The effect of clofibrate on decreasing serum bilirubin in healthy term
neonates under home phototherapy. Iran J Pediatr. 2010;20:48-52.
25. Penna F, Bonelli G, Baccino FM, Costelli P.
Cytotoxic properties of clofibrate and other peroxisome proliferators:
relevance to cancer progression. Curr Med Chem. 2010;17:309-20.
26. Loomba RS, Arora R. Prevention of cardiovascular
disease utilizing fibrates—a pooled meta-analysis. Am J Ther.
2010;17:e182-8.
27. Fidaleo M. Human health risk assessment for
peroxisome proliferators: more than 30 years of research. Exp Toxicol
Pathol. 2009;61:215-21.
|
|
|
 |
|

