|
|
|
Indian Pediatr 2010;47: 67-73 |
 |
Intermittent or Daily Short Course
Chemotherapy for Tuberculosis in Children: Meta-analysis of
Randomized Controlled Trials
|
|
P Ramesh Menon, R Lodha, S Sivanandan and SK Kabra
From the Department of Pediatrics, All India Institute of
Medical Sciences, New Delhi, India.
Correspondence to: SK Kabra, Professor, Department of
Pediatrics, All India Institute of Medical Sciences, New Delhi 110 029,
India.
Email: [email protected]
Received: November 11, 2008;
Initial review: December 11, 2008;
Accepted: February 10, 2009.
Published online 2009
May 20.
PII:S097475590800659-1
|
|
Abstract
Objective: To compare the effectiveness of
intermittent with daily chemotherapy (both containing rifampicin) in
childhood tuberculosis (age £16yrs)
in achieving cure/ significant improvement.
Design: Systematic Review and Meta-analysis.
Methods: MEDLINE and the Cochrane Library were
searched for randomized trials of antitubercular regimens containing
rifampicin, in children 16 yrs or less with tuberculosis. Two reviewers
independently assessed trial eligibility and quality. Data from full
articles of selected studies were independently extracted by two authors
and analyzed. The odds ratio was obtained for the pooled data in two
groups (intermittent and daily therapy).
Outcome variables: Cure/significant improvement,
relapse rate and adverse events.
Results: Four randomized controlled trials
comparing twice weekly and daily therapy including 466 children
(pulmonary 439; extrapulmonary 27) met the inclusion criteria. Baseline
data were comparable. On quality assessment, 3 studies scored 2 and one
study scored 3 out of 5 points. Per protocol analysis showed that
children receiving intermittent regimen were less likely to be cured
than those receiving daily therapy (OR 0.27; 95% CI: 0.14, 0.51). The
results of intention to treat analysis suggest similar trend towards
lower cure rates with twice weekly regimen (OR 0.66; 95% CI: 0.23-1.84).
Conclusion: Twice weekly intermittent short
course therapy is less likely to cure tuberculosis in children as
compared to daily therapy. There is a need for better quality randomized
controlled trials for assessing efficacy of alternate schedule for
intermittent therapy for childhood tuberculosis.
Key words: Children, Intermittent therapy, Short Course
chemotherapy, Treatment, Tuberculosis.
|
|
C
hildhood tuberculosis is a major
public health problem. There have been efforts to improve the treatment
and reduce the duration of therapy. DOTS was mainly introduced to improve
the adherence to therapy and cut the cost of the medicines used. Several
investigators in developing countries have found that high cure rates can
be achieved with rifampicin-containing intermittent regimens(1) in adult
patients with tuberculosis(2-4). Intermittent short course chemotherapy (SCC)
improves adherence to treatment and cure rates(3,5,6) and is
cost-effective(7). Intermittent therapy has been used in the National
Tuberculosis programs in two large countries (India, China) and recently
children suffering from tuberculosis have also been included as
beneficiaries. It is, therefore, relevant to evaluate the available
evidence on the efficacy of intermittent SCC in childhood tuberculosis.
We conducted a systematic review and meta-analysis of
studies comparing intermittent (with or without an initial period of daily
therapy) with daily short-course regimen (including rifampicin) in
children £16
years with tuberculosis in achieving cure/significant improvement.
Methods
Search strategy: We attempted to identify
all relevant studies without language restriction for the review. The
Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane
Library, Issue 4, 2008), Cochrane Database of Systematic Reviews were
searched. We combined the MEDLINE search (December 2008) with the highly
sensitive search strategy for identifying controlled trials, as designed
by Dickersin, et al.(8). The search terms used were: tuberculosis
(text or MESH heading), children (text or MESH heading), therapy (text or
MESH heading) with limits of age: 0-16 yrs, Randomized Controlled Trial.
All the citations were screened from titles. Of those considered to be
relevant, abstracts were screened. After screening of abstracts, full
articles were obtained if considered relevant. References of all the
studies were hand searched for potential inclusion. If a full article was
not available, the authors were contacted.
Inclusion criteria: Randomized controlled
trials (RCTs) in children aged 16 years or below with pulmonary/extrapulmonary
tuberculosis, which compared intermittent and daily regimen (containing
rifampicin), in hospital or ambulatory settings and recorded the outcome
of cure/significant improve-ment (symptomatic relief and/or radiologic
clearing at the completion of treatment course). Studies without any
separate data for children were excluded.
Data extraction: Two reviewers independently
extracted data pertaining to age, sex, history of contact with
tuberculosis patient, demonstration of acid fast bacilli (AFB) by
microscopy or culture, cure/significant improvement (symptomatic relief
and/or radiologic clearing at the completion of treatment course),
completion of treatment, relapse rate, adverse effects, and death.
Quality assessment: We used the
previously validated Jadad five point scale(9), to assess randomization
(zero to two points), double-blinding (zero to two points), and
withdrawals and dropouts (zero to one point).
Definitions used
Intermittent short course therapy: Any rifampicin-containing
multiple drug regimen, administered twice or thrice a week for a maximum
of nine months; initial daily dosing phase not exceeding one month.
Daily short course chemotherapy: Any rifampicin-containing
regimen given daily throughout (or five times a week in DOTS) for a
maximum of nine months.
Pulmonary TB: Children with TB involving at least
lungs (include disseminated tuberculosis with pulmonary involvement).
Extra pulmonary TB: Extra pulmonary tuberculosis
involving pleura, lymph nodes, abdomen, bones and joints, disseminated,
intestines, larynx, CNS.
Previously untreated patients: Patients who
did not receive antituberculous drugs in the past.
Smear positive: AFB demonstrated by Ziehl-Neelsen
stain on gastric lavage/ other fluid by direct microscopy.
Culture positive: Mycobacterium tuberculosis
identified on culture from gastric aspirate/ sputum or other body fluid.
Treatment completed: Children completing regimen
for assigned period.
Interrupted treatment: Children who interrupted
treatment for 2 months (8 weeks) of chemotherapy due to various reasons
(other than death).
Cure or significant improvement: A child who became
free of clinical symptoms and/or showed significant radiological
improvement at the end of assigned regimen.
Relapse rates: A child who showed cure/ significant
improvement with assigned regimen but had recurrence of symptoms during
follow up of up to 2 years.
Death: A child who died during the
chemotherapy.
Statistical analysis
Intention to treat and per protocol analysis were
performed using RevMan(4.2) program(10). Baseline data were compared.
Principal measure of effect of intervention (intermittent therapy) in
terms of cure, relapse, side effects and death was assessed using odds
ratio and 95% confidence interval. Random effects model was used in the
analysis, wherever required. Heterogeneity was assessed using I 2
test(10). Level of significance was chosen as P<0.05. To see the
impact of individual studies, sensitivity analysis was performed after
excluding one study at a time from the pooled data.
Results
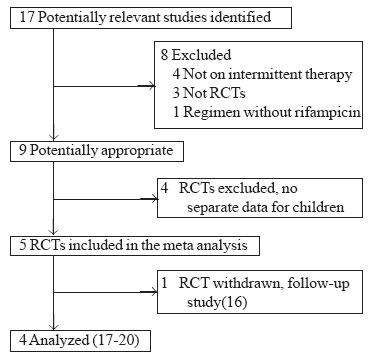
Figure 1 depicts the inclusion of trials
for meta-analysis. Four studies, enrolling 466 children met the inclusion
criteria for the systematic review(17-20). Table I
gives details of the 4 studies that fulfilled the inclusion criteria.
 |
|
Fig.1
Flow diagram of studies included for
meta-analysis.
|
TABLE I
Trials Comparing Intermittent Versus Daily
Therapy in Children with Tuberculosis
Baseline characteristics of patients:
There were no statistically significant differences in distribution by
age, sex distribution, nutritional status, history of contact with an
infective case of tuberculosis, BCG vaccination status, positive
tuberculin test and identification of acid fast bacilli between children
enrolled to receive either intermittent or daily therapy.
Type of tuberculosis: A total of 439
children (of 466) had pulmonary TB(PTB). The distribution of cases of PTB
in two groups were similar (P=0.67). One study enrolled ten cases
of cavitary tuberculosis(17). Only one study enrolled 27 children with
lymph-node tuberculosis with similar distribution between two groups (P=0.37)(19).
|
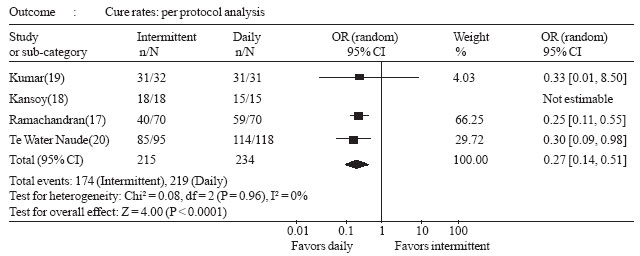 |
| Fig.2 Forest plot of cure
rates in intermittent versus daily chemotherapy for tuberculosis in
children. |
Cure/significant improvement: Per protocol
analysis revealed lower cure rates among children getting twice weekly
regimen as compared to daily regimen (OR 0.27, 95% CI 0.15-0.51 (Fig.
2). Results of intention to treat analysis suggested that there
was a trend towards lower cure rates in twice weekly intermittent therapy
as compared to daily therapy (OR 0.66; 95% CI 0.23-1.84). Results of
sensitivity analysis revealed that the cure rates were more in daily
treatment except when study by Ramachandran, et al.(17) is
excluded, but it did not reach statistical significance (Table
II). In study by Ramachandran, et al.(17), when only those who
did not need extended regimen were considered cured, the pooled analysis
shows trend towards better cure rates in daily regimen (OR 0.53, 95% CI
0.23-1.21), though it did not reach statistical significance.
TABLE II
Results of Sensitivity Analysis
|
Study excluded |
Cure rates (Cured/Total) |
OR (95% CI) |
| |
Twice weekly regimen |
Daily regimen |
|
| Kansoy, et al.(18) |
160/208 |
211/237 |
0.48 (0.20-1.17) |
| Kumar, et al.(19) |
142/185 |
188/210 |
0.48 (0.20-1.50) |
| Ramachandran, et al.(17) |
128/156 |
167/185 |
1.02 (0.29-3.55) |
| Te Water Naude, et al.(20) |
94/129 |
112/133 |
0.92 (0.17-5.05) |
Secondary outcomes: Table III
gives the details of the outcomes of included studies. In the study by
Kansoy, et al.(18), three children in daily treatment group were
excluded because of poor compliance. In the study by Kumar, et al.(19),
13 children were excluded: 10 children, who belonged to migrant farm
laborers, dropped out of the trial after a variable period of 2 to 4
months (interrupted treatment); all of them had pulmonary TB. Three
children, two of whom had died soon after completing 2 months of therapy
(reason not established) and one child who was diagnosed to have
Mycobacterium avium intracellulare infection were excluded from
analysis. Sixty-three children completed the study and had outcome
determined. In the study by Te Water Naude, et al.(20), treatment
records of seven children were described as lost for assessment of 4
weekly adherence data. However, the outcomes for these children have been
included for analysis and the authors have stated that "it is unlikely
that cases of relapse would not have come to our notice"(20).
TABLE III
Per Protocol Analysis for Treatment Outcome
| |
Kansoy, et al.(18) |
Ramachandran, et al.(17) |
Kumar, et al.(19) |
Te Water Naude, et al.(20) |
|
Regimen* |
I |
D |
I |
D |
I |
D |
I |
D |
|
Cure/ significant |
100% |
100% |
48% |
60% |
97% |
100% |
89% |
97% |
|
improvement |
(18/18) |
(15/15) |
(33/69) |
(41/68) |
(31/32) |
(31/31) |
(85/95) |
(114/118) |
|
Relapse |
0 |
0 |
0 |
1 |
0 |
0 |
1 |
0 |
|
Follow-up |
12 mo |
|
60 mo |
|
24 mo |
|
|
30 mo |
|
Adverse events |
Transaminitis (n=1) |
|
Jaundice (n=3) |
|
Vomiting (n=6);
joint pains (n=2) |
|
|
Vomiting |
|
* I = Intermittent; D =
Daily. |
The event rates for adherence, relapse, drug related
side effects and deaths were very low but there was no significant
difference between the two groups for these variables (P>0.05).
Only one study(19) reported data on children who interrupted treatment and
there was no difference between the groups (P=0.56).
Out of four studies, three scored 2 on Jadad’s 5 point
scale while one study scored 3 points suggesting that these studies were
not of very good quality.
Discussion
We identified and analyzed four RCTs including 466
children comparing intermittent, twice weekly therapy with daily therapy;
with mainly pulmonary tuberculosis. There was no RCT comparing the
efficacy of thrice weekly regimen with daily treatment in childhood
tuberculosis. Analysis of the pooled data revealed that daily therapy was
superior to twice weekly intermittent therapy for children.
The major problem in assessment of treatment outcome in
childhood tuberculosis is difficulty in defining outcome as documentation
of conversion from AFB positive to AFB negative state (that is the gold
standard for tuberculosis in adults) is extremely difficult as very small
proportion of patients are positive in the beginning. In such a scenario,
pediatricians have to rely on the clinico-radiologic markers of
improvement, this may lead to some subjectivity. We tried to define
outcome in the beginning and did multiple analysis (sensitivity analysis,
per protocol, intention to treat, and defining cure when the patient did
not require extension of treatment) to avoid bias in the results. All
analyses showed trend towards better outcome in daily treatment group as
compared to twice weekly regimen.
Directly observed therapy (DOT) may be conducted with
regimens given 3 times/week, or 5 times/week(22) or daily, with equal
efficacy depending on the drugs chosen. It is also postulated that
intermittent therapy may be even more effective than daily therapy (in
continuation phase) because it makes the organisms re-enter the phase of
multi-plication when the bactericidal drugs act best(23-24). Fewer doses,
even if they are larger, usually
reduce drug costs and may cause fewer side effects(7). A systematic review
comparing daily and intermittent antituberculosis regimen in adults(22)
found that there was no difference in cure rate (198 out of 199 in the
intermittent group compared to all 200 in the daily group), but 5 patients
relapsed in the group receiving intermittent therapy compared to one in
the group receiving daily regimen. A recent review on long term efficacy
of DOTS regimens for tuberculosis in adults concludes: "Although several
clinical trials supported the use of daily treatment regimens, studies
reporting tuberculosis recurrence after intermittent regimens were
limited. Overall there was wide variation in recurrence after successful
treatment, ranging from 0% to 14%. Considerable heterogeneity across
studies precluded the systematic assessment of factors contributing to
tuberculosis recurrence"(23).
The finding of inferiority of the intermittent regimen
in our review may be due to the different regimens for treatment. Two out
of the four studies had given twice weekly therapy during intensive phase
which is no longer recommended(24). In all the studies, the therapy in
intermittent regimen was directly observed, as recommended(25); even
though there was loss to follow up. A single missed dose in an
intermittent regimen represents a larger fraction of the total number of
treatment dose than in a daily regimen increasing the risk of treatment
failure. Adherence to therapy and hence, default rates may be influenced
by other factors (like overcrowding in household, default in the first
month in children with tuberculosis). One of the studies used a two drug
regimen of isoniazid and rifampicin for 9 months for daily therapy(17);
this will be considered as inadequate by current standards.
The main highlight of the review is that there are no
RCTs in children comparing thrice weekly with daily regimen. The available
studies comparing daily with twice weekly regimen lack uniformity in
diagnosis and assessment of outcome. To overcome heterogeneity in the
studies, we performed sensitivity analysis that revealed results in the
same direction except when study by Ramchandran, et al.(17) was
excluded. Even if it is presumed that in study by Ramachandran, et al.(17),
even those who did not need extension of treatment were cured, the pooled
analysis shows trend towards better cure rates in daily regimen. The study
has some limitations, the results may not be valid for extrapulmonary
tuberculosis as majority of the patient were having pulmonary TB. The data
on relapse, adherence and interruption were limited to few patients,
therefore, no valid conclusion can be drawn from this review for these
outcomes.
Contributors: SKK planned the study,
extracted data, conducted analysis and drafted manuscript and will act as
the guarantor. PRM searched literature, extracted data, and drafted
manuscript. RL conducted analysis and drafted manuscript. SS contributed
to literature search and manuscript writing.
Funding: None.
Competing interest: None stated.
|
What Is Already Known?
• Short course chemotherapy is effective in the
treatment of childhood tuberculosis.
What This Study Adds?
• Twice weekly intermittent therapy is inferior
to daily therapy in the treatment of childhood tuberculosis.
|
References
1. Cohn DL, Catlin BJ, Peterson KL, Judson FN, Sbarbaro
JA. A 62-dose, 6-month therapy for pulmonary and extrapulmonary
tuberculosis. A twice-weekly, directly observed and cost-effective
regimen. Ann Intern Med 1990; 112: 407-415.
2. Caminero JA, Pavón JM, Rodríguez de Castro F, Díaz
F, Julià G, Caylá JA, et al. Evaluation of a directly observed six
month fully intermittent treatment regimens for tuberculosis in patients
suspected of poor compliance. Thorax 1996; 51: 1130-1133.
3. Balasubramanian R. Fully intermittent six month
regimens for pulmonary tuberculosis in south India. Indian J Tuberc 1991;
38: 51.
4. Bechan S, Connolly C, Short GM, Standing E,
Wilkinson D. Directly observed therapy for tuberculosis given twice weekly
in the workplace in urban South Africa. Trans Royal Soc Trop Med Hyg 1997;
91: 704-707.
5. CDC core curriculum: treatment of TB Disease.
http://www.umdnj.edu/~ntbcweb/coretrea.htm. Accessed on 12 December, 2007.
6. Abernathy RS, Dutt AK, Stead WW, Moers DJ.
Short-course chemotherapy for tuberculosis in children. Pediatrics 1983;
72: 801-806.
7. Iseman MD, Cohn DL, Sbarbaro JA. Directly observed
treatment of tuberculosis - we can’t afford not to try it. N Engl J Med
1993; 328: 576-578.
8. Dickersin K, Scherer R, Lefebvre C. Systematic
reviews: Identifying relevant studies for systematic reviews. BMJ 1994;
309:1286-1291.
9. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds
DJ, Gavaghan DJ, et al. Assessing the quality of reports of
Randomized clinical trials: is blinding necessary? Controlled Clin Trials
1996; 17:1-12.
10. RevMan Analyses [Computer program]. In:
Review Manager (RevMan). Version 4.2 for Windows. Copenhagen: The Nordic
Cochrane Centre, The Cochrane Collaboration; 2003.
11. Dingley HB. Short-term chemotherapy in tuberculosis
in children. Indian J Tuberc 1982; 29: 48-54.
12. Jawahar MS, Rajaram K, Sivasubramanian S,
Paramasivan CN, Chandrasekar K, Kamaludeen MN, et al. Treatment of lymph
node tuberculosis—a randomized clinical trial of two 6-month regi-mens.
Trop Med Int Health 2005; 10: 1090-1098.
13. Rajeswari R, Sivasubramanian S, Balambal R,
Parthasarathy R, Ranjani R, Santha T, et al. A controlled clinical
trial of short-course chemotherapy for tuberculoma of the brain. Tuber
Lung Dis 1995; 76: 311-317.
14. Balasubramanian R, Nagarajan M, Balambal R,
Tripathy SP, Sundararaman R, Venkatesan P, et al. Randomised
controlled clinical trial of short course chemotherapy in abdominal
tuberculosis: a five-year report. Int J Tuberc Lung Dis 1997; 1: 44-51.
15. Anonymous. Controlled clinical trial of oral
short-course regimens in the treatment of sputum-positive pulmonary
tuberculosis. Tuberculosis Research Centre. Int J Tuberc Lung Dis 1997; 1:
509-517.
16. Swaminathan S, Raghavan A, Duraipandian M,
Kripasankar AS, Ramachandran P. Short course chemotherapy for pediatric
respiratory tuberculosis: 5-year report. Int J Tuberc Lung Dis 2005; 9:
693-696.
17. Ramachandran P, Kripasankar AS, Duraipandian M.
Short Course Chemotherapy for pulmonary tuberculosis in children. Indian J
Tuberc 1998; 45: 83-87.
18. Kansoy S, Kurtaþ N, Akþit S, Aksoylar S, Yaprak I,
Çaðlayan S. Superiority of intermittent-short course chemotherapy in
childhood pulmonary tuberculosis. Turkish J Med Sci 1996; 26: 41-43.
19. Kumar L, Dhand R, Singhi PD, Rao KL, Katariya S. A
randomised trial of fully intermittent and daily followed by intermittent
short-course chemotherapy for childhood tuberculosis. Pediatr Infect Dis J
1990; 9: 802-806.
20. Te Water Naude JM, Donald PR, Hussey GD, Kibel MA,
Louw A, Perkins DR, et al. Twice-weekly vs daily chemotherapy for
childhood TB. Pediatr Infect Dis J 2000; 19: 405-410.
21. World Health Organization. Provisional guidelines
for the diagnosis and classification of the EPI target diseases for
primary health care, surveillance and special studies. EPI/GEN/83/4.
Geneva: WHO; 1983.
22. Mwandumba HC, Squire SB. Fully intermittent dosing
with drugs for treating tuberculosis in adults. Cochrane Database Syst Rev
2001; 4: CD000970.
23. Cox HS, Morrow M, Deutschmann PW. Long term
efficacy of DOTS regimens for tuberculosis: systematic review. BMJ 2008;
336: 484-487.
24. Walley JD, Khan MA, Newell JN, Khan MH.
Effectiveness of the direct observation component of DOTS for
tuberculosis: a randomized controlled trial in Pakistan. Lancet 2001; 357:
664-669.
25. Snider DE, Graczyk J, Bek E, Rogowski J. Supervised
six-months treatment of newly diagnosed pulmonary tuberculosis using
isoniazid, rifampin, and pyrazinamide with and without streptomycin. Am
Rev Respir Dis 1984; 130: 1091-1094.
|
|
|
 |
|

