|
|
|
Indian Pediatr 2009;46: 35-47 |
 |
Management of Steroid Resistant Nephrotic
Syndrome |
|
Indian Society of Pediatric Nephrology
Correspondence to: Dr. Arvind Bagga, Department of
Pediatrics,
All India Institute of Medical Sciences, Ansari Nagar,
New Delhi 110 029, India.
E-mail: [email protected]
|
|
Abstract
Justification: There is a lack of evidence based
guidelines for management of children with steroid resistant nephrotic
syndrome (SRNS).
Process: Experts of the Indian Society of Pediatric
Nephrology were involved in a two-stage process, the Delphi method
followed by a structured face to face meeting, to formulate guidelines,
based on current practices and available evidence, on management of
these children. Agreement of at least 80% participants formed an opinion.
Objectives: To develop specific, realistic,
evidence based criteria for management of children with idiopathic SRNS.
Recommendations: The Expert Group emphasized that
while all patients with SRNS should initially be referred to a pediatric
nephrologist for evaluation, the subsequent care might be collaborative
involving the primary pediatrician and the nephrologist. Following the
diagnosis of SRNS (lack of remission despite treatment with prednisolone
at 2 mg/kg/day for 4 weeks), all patients (with initial or late
resistance) should undergo a renal biopsy, before instituting specific
treatment. Patients with idiopathic SRNS secondary to minimal change
disease or focal segmental glomerulosclerosis should receive similar
therapy. Effective regimens include treatment with calcineurin inhibitors
(tacrolimus, cyclosporine), intra-venous cyclophosphamide or a combination
of pulse corticosteroids with oral cyclophosphamide, and tapering doses of
alternate day corticosteroids. Supportive management comprises of, when
indicated, therapy with angiotensin converting enzyme inhibitors and
statins. It is expected that these guidelines shall enable standardization
of care for patients with SRNS in the country.
Keywords: Cyclosporine, Delphi method, Nephrotic syndrome,
Tacrolimus. |
|
Idiopathic nephrotic syndrome,
characterized by altered permselectivity of the glomerular filter, is a
common chronic renal disorder in children. Most patients are steroid
sensitive and respond to therapy with remission of proteinuria (steroid
sensitive nephrotic syndrome). Revised Guidelines for treatment of these
patients were published recently(1). Approximately 10-20% children with
nephrotic syndrome, who do not respond to therapy with corticosteroids,
are classified as steroid resistant (SRNS). Their management is difficult
since patients are, on one hand, at risk for complications of unremitting
nephrotic syndrome and progressive renal disease and on the other, the
side effects of treatment with immunosuppressive medications(2).
Despite the availability of a number of agents with
variable efficacy in inducing remission, the optimal treatment of patients
with SRNS is unclear. A lack of controlled studies has hindered
development of guidelines on treatment. In order to address the management
of this condition, we used the Delphi technique to gather opinion of
experts of the Indian Society of Pediatric Nephrology. This technique
comprises a series of questionnaires, which are circulated among experts
followed by, if necessary, a face-to-face meeting to enable consensus on
issues where evidence based recommendations are lacking(3). Such an
approach has been used to develop consensus on the management of juvenile
arthritis and classification of childhood vasculitides(4).
Objectives
Experts of the Indian Society of Pediatric Nephrology
were involved in a two-stage process to formulate broad guidelines for the
management of patients with idiopathic SRNS.
Methods
The first stage comprised the Delphi method to generate
responses via electronic mail. This was followed by a structured
face-to-face meeting to facilitate discussion on issues related to the
topic.
The Delphi method
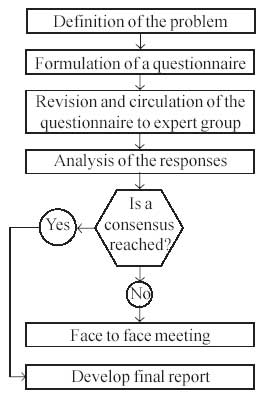
The methodology was designed such that each step was
based on the results of the previous steps (Fig.
1).
Step 1. Definition of the problem
There is a lack of evidence based guidelines on
management of SRNS in children.
Step 2. Formulation of a questionnaire
A local committee designed a questionnaire comprising
26 issues related to management of children with SRNS. The
questionnaire was in a multiple-choice format, each choice being rated on
a scale of 0 to 7. The questionnaire was circulated electronically among
members of the local committee to elicit their response on its
suitability.
Step 3. Revision and circulation of the questionnaire
Based on responses of the local committee, the
questionnaire was revised and circulated to 33 experts across the
country by electronic mail. At this stage, the participants were provided
with literature on management of patients with SRNS.
Step 4. Analysis of the responses
Responses, obtained from 31 pediatric nephrologists,
were collated; choices with a score of six or more in at least 80% of the
responses formed an opinion. This was possible in 15 of the 26 questions
circulated. An opinion was not possible in the remaining questions.
 |
|
Fig 1. Delphi method
for formulating guidelines for management of steroid resistant
nephrotic syndrome. |
Face-to-face meeting
Experts of the Indian Society of Pediatric Nephrology (Annexure
I) met on 16 November 2007 in Hyderabad, to review each of the issues
and formulate recommendations based on opinion derived from the previous
phase and current medical literature. Agreement of at least 80%
participants was taken as a recommendation.
Grading recommendations
Wherever possible, treatment recommendations were
graded from A to D (Table I) based on the level of
available evidence, as proposed by Carruthers, et al.(5).
TABLE I
Levels of Evidence for Rating Studies and Grading for Treatment Recommendations*
| Level |
Definition of evidence |
| 1 |
Randomized controlled trial (RCT) that demonstrates
a statistically significant difference in at least one important
outcome, or if the difference is not significant, an RCT of adequate
size to exclude a 25% difference in relative risk with 80% power,
given the observed results |
| 2 |
RCT that does not meet level 1 criteria |
| 3 |
Non-randomized trial with contemporaneous controls
selected by some systematic method, or sub-group analysis of a RCT |
| 4 |
Before-after study or case series (>10 patients)
with historical controls, or controls drawn from other studies |
| 5 |
Case series (>10 patients) without controls |
| 6 |
Case reports (<10 patients) |
| Grading |
Definition of recommendation |
| A |
Recommendation based on one or more studies at Level
1 |
| B |
Best level of evidence available was at Level 2 |
| C |
Best level of evidence available was at Level 3 |
| D |
Best level of evidence available was lower than
Level 3 and included expert opinion |
* Reproduced with permission of the publisher. © 1993 Canadian Medical Association(6)
Recommendations
In view of its rarity, complexity of treatment,
progressive course and unsatisfactory outcome, all patients with SRNS
should be referred to a pediatric nephrologist for evaluation.
Subsequently, the care of these patients might be collaborative, between
the primary pediatrician and the nephrologist.
1. Definitions
(a) A patient is diagnosed to have steroid
resistance if there is lack of remission despite treatment with
prednisolone at a dose of 2 mg/kg/day (60 mg/m 2/day)
for 4 weeks. Remission is defined as absence of proteinuria
(urine albumin nil or trace for three consecutive days by dipstick or
boiling test).
(b) Even in patients with adverse effects
related to previous steroid use, confirmation of lack of remission
despite 4 weeks’ treatment with daily prednisolone is necessary before
making the diagnosis of SRNS.
(c) Similar definitions for duration of
steroid therapy should be used for initial and late steroid resistance.
Initial resistance is lack of remission at the first episode of
nephrotic syndrome. Patients who are steroid sensitive initially, but
show steroid resistance during a subsequent relapse have late
resistance.
Rationale
Following treatment with daily prednisolone, 95%
patients with steroid sensitive nephrotic syndrome achieve remission by
the first 4 weeks and an additional 3% in additional 4 weeks(6). Prolonged
courses of daily corticosteroids are associated with increased incidence
of side effects. Therefore, defining SRNS as lack of remission despite 4
weeks treatment with daily prednisolone is reasonable. This definition is
in conformity with that used by the Cochrane Renal Group(7). The National
Institutes of Health (USA) trial on patients with steroid resistant focal
segmental glomerulosclerosis (FSGS) has accepted a similar definition (www.fsgstrial.org).
Absence of proteinuria by dipstick usually correlates with a spot urinary
protein to creatinine ratio less than 0.2 mg/mg. Since systemic infections
(e.g., peritonitis, cellulitis, respiratory tract infections) might
result in persistent proteinuria and an incorrect diagnosis of SRNS, these
should be carefully excluded.
2. Renal Biopsy
(a) All children with SRNS, whether initial or
late, should undergo a renal biopsy before instituting specific
treatment.
(b) The histological specimen must be examined
by light and immunofluorescence microscopy. Referral centers should
develop facilities for electron microscopic evaluation of renal biopsy
specimens.
Rationale
Despite absence of evidence based recommendations
regarding the role of renal biopsy in patients with SRNS, this procedure
provides important information on renal histology and outcome. Most
patients with steroid sensitive nephrotic syndrome (90%) show minimal
change nephrotic syndrome on renal histology. The renal histology in SRNS
is different, with 30-40% patients each showing minimal change nephrotic
syndrome and FSGS, and a smaller group with mesangio-proliferative
glomerulonephritis(8). The response to therapy is determined by renal
histology; patients with minimal change nephrotic syndrome show
satisfactory response to therapy, while presence of FSGS or chronic
tubulointerstitial changes is associated with unsatisfactory outcomes(9).
A renal biopsy is also necessary before initiating treatment with
potentially nephrotoxic agents, especially cyclosporine or tacrolimus(10).
Approximately 20% patients with SRNS show
membranoproliferative glomerulonephritis, memb-ranous nephropathy, IgA
nephropathy and amyloidosis. Recognition of these conditions is important
as they differ with regard to their evaluation and treatment.
Although light and immunofluorescence microscopy form
the minimum requirement for evaluation of histopathology specimens,
electron microscopy helps to confirm the diagnosis of minimal change
nephrotic syndrome, differentiates primary from secondary FSGS, and
enables diagnosis of early membranous nephropathy, membranoproliferative
glomerulonephritis and Alport syndrome.
3. Mutational Analysis
(a) Studies for mutations of genes involved in
synthesis of podocyte proteins are not routinely necessary in children
with SRNS.
(b) Where facilities exist, mutational
analysis may be offered to patients with congenital nephrotic syndrome
(onset below 3 months of age), initial steroid resistance and family
history of SRNS.
Rationale
Mutations in the genes encoding various podocyte
proteins, including podocin (NPHS2) and nephrin (NPHS1),
have been described in a variable proportion of patients with familial and
sporadic SRNS(11). The likelihood of detecting a mutation is higher in
patients with family history of nephrotic syndrome or its onset in
infancy(12). Patients with mutations involving these genes often do not
respond to immunosuppressive medications and show progressive kidney
disease. In a series of patients with SRNS and homozygous or compound
heterozygous mutations in NPHS2, none showed complete remission
following treatment with cyclophosphamide or cyclosporine(13). Mutations
of the gene encoding Wilms’ tumor protein (WT1) may result in a
phenotype comprising FSGS, pseudohermaphroditism and increased risk for
renal or gonadal malignancies(14). Finally, while 30% patients of FSGS
without mutations show a recurrence of the disease post-transplant, this
is exceptionally rare in patients with mutations in the above genes(13).
In view of lack of data in Indian children, routine
mutational analysis in patients with initial SRNS is not recommended.
Patients with late steroid resistance have not been found to have genetic
mutations(15). The utility of mutational studies prior to instituting
therapy with alternative agents is also unclear.
4. Principles of Therapy
Patients with idiopathic SRNS secondary to minimal
change nephrotic syndrome, FSGS and mesangioproliferative
glomerulonephritis should receive similar therapy.
Rationale
Review of the literature suggests that patients with
steroid resistance secondary to minimal change nephrotic syndrome are more
likely to achieve remission and have a better prognosis compared to other
histological types(9,16,17). However, a systematic review by the Cochrane
Renal Group showed similar outcome in patients with steroid resistant
minimal change nephrotic syndrome and FSGS who were treated with
cyclosporine or cyclophosphamide(7). There is no clear evidence to support
that patients with minimal change nephrotic syndrome and FSGS should be
treated differently.
Distinction between various histological categories is
also not absolute. In early stages, FSGS might be difficult to distinguish
from minimal change nephrotic syndrome, depending on the adequacy of
biopsy and extent of the disease. Furthermore, examination of renal
histology in FSGS reveals a variety of histological subtypes, with
variable response to therapy and outcome(18). Repeat biopsies might show
morphological transition between minimal change nephrotic syndrome,
mesangioproliferative glomerulo-nephritis and FSGS. Thus, these
histological conditions may be found alone or in combination on sequential
biopsies in the same patient. Finally, studies in adults suggest that the
chief factor predicting outcome is the response of proteinuria to therapy
rather than the renal histology(19).
5. Specific Treatment
The aim of therapy is induction of remission while
avoiding medication related toxicity. Treatment failure correlates with
poor long-term prognosis for renal function. In view of limited studies in
children with SRNS, treatment guidelines vary considerably and there is
absence of consensus on therapy.
TABLE II
Regimens for Treatment of Idiopathic Steroid Resistant Nephrotic Syndrome
|
Drug |
Dosage* |
Remission |
|
Calcineurin inhibitors |
| Cyclosporine and prednisolone** |
4-6 mg/kg/day in two divided doses for 2-3 years |
50-80% |
| Tacrolimus and prednisolone** |
0.12-0.15 mg/kg/day in two divided doses for 2-3 years |
70-85% |
| Cyclophosphamide |
| Oral cyclophosphamide and prednisolone** |
2-3 mg/kg/day for 12 weeks |
25-30% |
| IV cyclophosphamide and prednisolone** |
500-750 mg /m2 once every month for 6 months |
40-65% |
| Pulse corticosteroids |
| IV methylprednisolone, oral cyclophosphamide
and prednisolone# |
20-30 mg/kg for 6 alternate day pulses; then once a
week for 8 doses, fortnightly for 4 doses, once a month for
8 doses; finally bimonthly for 4 doses |
40-70% |
| IV dexamethasone, oral cyclophosphamide and prednisolone# |
4-5 mg/kg for 6 alternate day pulses; then every
fortnight for 4 doses; finally once a month for 8 doses |
30-50% |
* Dosage refers to that of the italicized agent;
** Prednisolone dose: 1.5 mg/kg on alternate days for 4 weeks;
1.25 mg/kg next 4 weeks; 1 mg/kg for 4 months;
0.5-0.75 mg/kg for 12-18 months;
# Oral cyclophosphamide for 12 weeks (weeks 3-15);
tapering doses of prednisolone over 12 months
Effective regimens and their side effects are shown in
Tables II and III. The options for
treatment for patients with idiopathic SRNS include:
• Calcineurin inhibitors with tapering doses of
alternate day steroids: cyclosporine (Grade A recommendation);
tacrolimus (Grade D recommendation)
• Cyclophosphamide with tapering doses of alternate
day steroids (Grade C recommendation)
• High dose intravenous steroids (dexamethasone,
methylprednisolone) with oral cyclophos-phamide and tapering alternate
day steroids (Grade C recommendation)
In view of lack of consensus regarding the most
appropriate therapy, the Expert Group accepts that the choice of initial
treatment shall continue to depend on the preference of the physician and
the cost of medications.
TABLE III
Common Side Effects of Medications Used for Treatment
| Drug |
Common side effects |
| Cyclophosphamide |
Alopecia, marrow suppression, vomiting,
hemorrhagic cystitis, risk of systemic infections |
| IV methylprednisolone, |
Hypertension, hypokalemia,
dexamethasone, hyperglycemia, steroid psychosis, risk of systemic
infections |
| Cyclosporine, Tacrolimus |
Nephrotoxicity; hypertension;
hypertrichosis, gingival hyper-plasia and dyslipidemia*;
neurotoxicity, diarrhea and hyperglycemia** |
| ACE inhibitors e.g., |
Dry cough, hyperkalemia, enalapril
anemia |
| Statins e.g., atorvastatin |
Headache, muscle pain, rash, raised
transaminases |
Side effects frequent with *cyclosporine or **tacrolimus
Rationale
There is a lack of consensus on the most appropriate
first line therapy for children with SRNS, with many of the regimens
extrapolated from studies in adults. The level of evidence(5) on
efficacies of available regimens is summarized below.
Calcineurin inhibitors
Cyclosporine has been compared to placebo, control or
supportive treatment in three randomized trials(20-22). Treatment
significantly increased the proportion of patients who achieved complete
remission compared with placebo or no treatment, irrespective of renal
pathology [three studies, n=49; relative risk (RR) 0.64, 95%
confidence interval (CI) 0.47, 0.88]. While no patient achieved complete
remission in one study, urinary protein excretion and creatinine clearance
worsened significantly in the control group (Level 2)(20). The other two
trials showed significant benefit in terms of proportion of children who
achieved either complete or partial remission (Level 1)(21,22). Relapse
was reported in 33.3% children, who achieved partial or complete
remission, by the end of 12 months’ treatment(22). No data was shown on
differences in efficacy in patients with initial compared to late
resistance, or on long term effect on renal function.
A meta-analysis of these studies shows that treatment
with cyclosporine results in a significant increase in the number of
children (both minimal change nephrotic syndrome and FSGS) with complete
remission compared to placebo or supportive treatment (RR 7.66, 95% CI
1.1, 55.3)(7). These data confirm the findings of multiple uncontrolled
studies on the role of cyclosporine in patients with SRNS. A case series
of 65 patients with initial steroid resistance showed complete remission
in 46% with minimal change nephrotic syndrome and 30% with FSGS (Level
4)(23). Another retrospective report showed remission in 77% of 51
patients with FSGS treated with cyclosporine and prednisone, with or
without intravenous methylprednisolone(17).
There is limited data on the efficacy of tacrolimus,
which has a similar mode of action as cyclosporine (Level 5)(24). A
randomized controlled trial, published in abstract form, reported similar
remission rates with these agents (Level 2)(25). Tacrolimus has an
advantage of a better side effect profile with less cosmetic side effects
but the incidence of neurotoxicity and impaired glucose tolerance appear
greater. In all published trials, the incidence of adverse effects was
low, but this might be underestimated because of small patient numbers,
short follow up periods and incomplete reporting.
Cyclophosphamide
Three randomized controlled trials have investigated
the role of cyclophosphamide(26-28). Of these, two studies compared oral
cyclophosphamide and alternate day prednisolone with prednisolone
alone(26,27). There was no difference in the number of children overall (n=84;
RR 1.01, 95% CI 0.74, 1.36) or those with FSGS (n=63; RR 0.82, 95%
CI 0.46, 1.49) who achieved complete or partial remission following
treatment with cyclophos-phamide (Level 2; no benefit demonstrated). The
proportion of patients with renal function deterioration (one study, n=60;
RR 1.59, 95% CI 0.87, 2.88) or who died (RR 1.07, 95% CI 0.19 to 5.95) did
not differ between the two groups. However, the mean interval between
onset of treatment and time to response was shorter with cyclophosphamide
plus prednisolone compared with prednisolone alone [38.4 days (range 6-80
days) versus 95.5 days (range 61-129), P<0.05]. While no statistically
significant benefits of treatment were found, the number of patients
studied was small and a beneficial effect of oral cyclophosphamide in SRNS
cannot be excluded. Prospective studies are necessary to examine whether
therapy with oral cyclophosphamide and prednisolone might be effective in
a subgroup of patients with SRNS.
A study with few subjects, which compared intravenous
with oral cyclophosphamide in minimal change nephrotic syndrome found that
all 7 patients in the IV group had remission, compared with one of four in
the latter; differences between the groups were not significant (n=11;
RR 0.09, 95% CI 0.01, 1.39) (Level 2)(28). A number of case series have
examined the role of intravenous cyclophosphamide, administered monthly
for six doses along with tapering doses of alternate day prednisolone.
Review of this data suggests that therapy results in remission in 40-65%
patients(29).
High dose glucocorticoids and oral cyclophosphamide
A non-randomized trial on patients with FSGS, comparing
6-months to 18-months regimen of intravenous methylprednisolone, showed
remission in 60% and 85.7% patients respectively (Level 3)(30).
Multiple case series, combining intravenous
corticosteroids, oral alkylating agents and prednisolone, show remission
in 30-70% cases (Level 4)(31). A significant proportion of
patients receiving treatment with this intensive regimen are at risk for
complications, including systemic infections, hypertension and electrolyte
abnormalities. In view of the risks of steroid toxicity and the need for
multiple hospitalizations, extended protocols have been replaced by
abbreviated regimens utilizing fewer doses of intravenous corticosteroids
(Table II).
While the commonly used agent for intravenous therapy
is methylprednisolone, a prospective study comparing intravenous
dexamethasone to methylprednisolone showed no difference in terms of short
term efficacy or adverse effects(32). Dexamethasone and methylprednisolone
showed similar respective rates of complete remission (35.1%, 95% CI 22.9,
48.9; and 33.3%, 95% CI 14.6, 46.9) (Level 3). The median time to response
was similar at 10 days and the most common side effect was hypertension.
Comparative studies
Two recently published randomized controlled trials
have compared the relative efficacy of the therapies, discussed above. The
first study compared treatment with intravenous cyclophosphamide and oral
prednisolone with oral cyclophosphamide, intra-venous dexamethasone and
oral prednisolone in 49 patients with SRNS(33). At 6-months, the
respective rates of complete remission were comparable at 53.8% and 47.8%
(Level 2). Patients in both groups showed a high risk of infections; other
adverse effects included cushingoid features, hypertension, hypokalemia,
vomiting and reversible alopecia.
The Arbeitsgemeinschaft fur Padiatrische Nephrologie
(APN) recently reported the results of a multicenter randomized controlled
trial on therapy with oral cyclosporine (150 mg/m 2/day
for 48 weeks) versus intravenous cyclophosphamide (500 mg/m2; seven
doses over 36 weeks) in 32 patients with initial SRNS(34). While the rates
of complete remission were low in both groups, significantly more patients
treated with cyclosporine (7/15; 46.7%) compared with cyclophosphamide
(1/17; 5.9%) had partial remission (P=0.013) (Level 1). Similar
findings were described in a retrospective analysis of 37 adult patients
with SRNS (histology showing minimal change nephrotic syndrome, FSGS and
mesangioproliferative glomerulonephritis) who received treatment with
either intravenous cyclophosphamide or cyclosporine(35). At 12 months, the
efficacy of the two treatment regimens was 40% and 85.7% respectively
(Level 4). A recent report published in abstract form showed significantly
higher remission rates with oral tacrolimus and prednisolone as compared
to pulse intravenous cyclophosphamide and prednisolone in Chinese adults
with idiopathic steroid-resistant minimal change disease(36). While
results from these trials suggest that calcineurin inhibitors should be
considered as the first line therapy for patents with initial steroid
resistance, these findings need confirmation in a larger number of
patients and with extended follow up.
The number of treatment regimes in practice is a
testimony to a lack of consensus in managing these heterogeneous groups of
patients. Most experience is derived from case series and anecdotal
reports, rather than being based on prospective randomized controlled
trials. The results of treatment using intravenous cyclophosphamide are
promising but require confirmation. Treatment with pulse corticosteroids,
oral cyclophosphamide and prednisolone is effective in a proportion of
patients, but the high incidence of adverse effects limits its overall
benefits. While benefits following treatment with calcineurin inhibitors
(cyclosporine or tacrolimus) with alternate day prednisolone are
increasingly evidence based, there is limited data on long term renal
function. The need for prolonged treatment and risk of nephrotoxicity
limit the widespread use of these agents. Finally, a proportion of
patients failing to respond to a particular regimen might show remission
following treatment with alternative agents.
Other agents
Other agents that have been used anecdotally are
vincristine, mycophenolate mofetil(37), plasma-pheresis(38) and
rituximab(39).
6. Dose and Duration of Treatment
Guidelines on dose and duration of treatment with
various agents are summarized in Table II.
There is a lack of guidelines on duration of treatment
with calcineurin inhibitors. Most patients who respond to treatment do so
within 2-3 months of initiating therapy. Therapy should therefore be
considered not effective and discontinued in patients showing persistent
nephrotic range proteinuria beyond 6 months. On the other hand, those
showing complete or partial remission should receive treatment for 2-3
years; the dose of calcineurin inhibitors is tapered to the lowest
effective dose for another 1-2 years. While there are reports on
successful switching of treatment from calcineurin inhibitors to
mycophenolate mofetil, the long-term benefits of such a strategy need
confirmation(40). A proportion of patients who respond to treatment with
cyclosporine relapse on its discontinuation; reintroduction of therapy is
not always effective.
7. Monitoring Response to Therapy
Patients should be monitored initially every month,
then every 3-4 months. Response to therapy is categorized as complete or
partial remission of proteinuria. Complete remission is defined as
presence of trace or negative proteinuria (by dipstick test) or spot urine
protein to creatinine ratio (Up/Uc) <0.2 mg/mg. Patients are considered to
be in partial remission if they show 1-2+ proteinuria (or Up/Uc
between 0.2-2), serum albumin >2.5 g/dL and no edema. Non-response
is defined as 3-4+ proteinuria (or Up/Uc >2), serum albumin <2.5 g/dL or
edema. While the aim of treatment is achievement of complete remission,
the occurrence of partial remission is satisfactory(41).
8. Choice of Calcineurin Inhibitor and Monitoring of
Therapy
(a) The aim of treatment with
calcineurin inhibitors is achievement of complete or partial remission
and long-term preservation of glomerular filtration rate to within 20%
of pretreatment value.
(b) In view of similar efficacy and
less cosmetic toxicity, treatment with tacrolimus is preferred to
cyclosporine, especially in girls. A factor limiting the use of
tacrolimus in very small children is the non-availability of drug in
liquid form.
(c) Blood levels of cyclosporine or
tacrolimus should be routinely measured once, 2-4 weeks following
initiation of therapy. Subsequent determination of levels is necessary
in case of suspected drug toxicity or if the patient is receiving
medications that might affect levels of these agents. Trough (12-hr)
blood levels of cyclosporine should be maintained at 80-120 ng/mL and
tacrolimus at 5-8 ng/mL.
(d) Prolonged therapy with calcineurin
inhibitors might be associated with histological features of
nephrotoxicity, without elevation of blood levels of serum creatinine.
Renal biopsy is therefore necessary following 2-3 years of therapy to
evaluate for nephrotoxicity. Examination of renal histology is also
informative in patients with declining renal function (serum creatinine
>50% above baseline), which persists despite reduction in dose or
discontinuation of treatment with these agents.
Rationale
There is limited evidence to support the superiority of
tacrolimus over cyclosporine in patients with nephrotic syndrome. Results
from case series and a randomized controlled trial suggest that tacrolimus
is similar in efficacy to cyclosporine but with less cosmetic side effects
and decreased incidence of dyslipidemia(25). Estimation of trough blood
levels is recommended for monitoring. While it is proposed that second
hour measurement of cyclosporine (C2) may be a better predictor than
trough levels (Co) in patients with nephrotic syndrome, the former targets
are yet to be defined(42). Trough levels of tacrolimus have been used to
monitor renal transplant recipients and a similar strategy can be applied
to patients with nephrotic syndrome.
In view of a lack of correlation between serum
creatinine and severity of histological changes, renal biopsies are
recommended in patients receiving long term (>2 years) therapy with these
agents(40, 43). Histological features suggesting acute nephro-toxicity
include necrosis and hyaline deposition in individual myocytes, isometric
vacuolation in tubular cells, endothelial vacuolation, afferent
arteriolopathy and rarely thrombotic microangiopathy(10). Chronic changes
comprise nodular hyalinosis, segmental or global glomerular sclerosis or
striped interstitial fibrosis and tubular atrophy. Risk factors for
nephrotoxicity include prolonged duration of cyclosporine therapy (3
mg/kg/day, for more than 24 months) and persistence of heavy proteinuria
beyond 30 days. The presence of increasing fibrosis should lead to a
careful review, since this might be the result of calcineurin inhibitor
toxicity or progression of glomerular disease. The decision to lower or
discontinue medication or add adjunctive therapy is based on clinical
course and histological changes.
9. Angiotensin Converting Enzyme Inhibitors and
Angiotensin Receptor Blockers
(a) All patients with SRNS should
receive treatment with angiotensin converting enzyme inhibitors (e.g.,
enalapril, ramipril), initially at low dose; later the dose may be
increased based on the severity of proteinuria (Grade C recommendation).
(b) These agents should be avoided if
the estimated GFR is <30 ml/minute/1.73 m 2.
(c) Angiotensin receptor blockers (e.g.,
losartan, valsartan) may be used in patients intolerant to
angiotensin converting enzyme inhibitors, or as add-on therapy to
achieve better antihypertensive and antiproteinuric effect (Grade D
recommendation).
Rationale
There is evidence to support the antiproteinuric and
renoprotective effects of angiotensin converting enzyme inhibitors. In a
controlled trial, fosinopril significantly reduced proteinuria and
alleviated renal tubular damage, but did not influence blood pressure in
normotensive children with SRNS (Level 3)(44). In another randomized
controlled study, the antiproteinuric effect was lower with enalapril
given at low dose (0.2 mg/kg/d) (median reduction 34.8%; 95% CI -7.9,
76.6) compared to high dosage (0.6 mg/kg/d) (reduction 62.9%; 95% CI 40.6,
71.6) (Level 2)(45). Although studies in adults (Level 1)(46) recommend
the combined use of angiotensin receptor blockers with angiotensin
converting enzyme inhibitors to potentiate the antiproteinuric effects,
there is paucity of data on the efficacy and safety of combined therapy in
children.
10. Lipid Profile and Use of Medications
(a) Lipid profile [total cholesterol,
low-density lipoproteins (LDL), very low-density lipoproteins (VLDL) and
triglycerides (TG)] should be done annually in patients with SRNS (Grade
D recommendation).
(b) The indications for starting
therapy are an aberration in the lipid profile, which persists despite
3-6 months of specific treatment. Patients with blood levels of total
cholesterol >200 mg/dL, LDL cholesterol >130 mg/dL and triglycerides
>200 mg/dL require therapy. Although evidence based guidelines are
lacking for children, therapy with HMG CoA reductase inhibitors (e.g.
atorvastatin) is recommended.
Rationale
Persistent dyslipidemia is an important risk factor for
the occurrence of cardiovascular disease. In view of limited pediatric
data, the above targets are in accordance with those proposed for adults.
The target LDL level has been set as <130 mg/dL as suggested by the Kidney
Disease Outcome Quality Initiative (KDOQI) for adolescents with chronic
kidney disease(47). There is evidence that control of dyslipidemia leads
to control of proteinuria and regression of renal fat deposits
(Level 4)(48). Long-term studies are necessary to assess the beneficial
effects of lipid lowering on renal histology and disease
progression.
Comments
Guidelines on the evaluation and management of patients
with steroid sensitive nephrotic syndrome were revised recently(1). The
treatment of patients with SRNS continues to be challenging. The above
recommendations, based on expert opinion and published evidence, are
intended to provide guidelines on management for these patients.
Consensus was achieved on the definition of SRNS and
role of histopathology and genetic studies in these patients. There was
agreement on the need for adequate supportive therapy comprising ACE
inhibitors, antihypertensive and lipid lowering agents. The need for
careful clinical and biochemical monitoring was emphasized.
However, a lack of controlled trials has resulted in
absence of consensus on the specific management of these patients. The
number of immunosuppressive regimens proposed is an acknowledgement of the
lack of satisfactory treatment for these patients. Accepting this
limitation, the Expert Group proposed that this statement provide details
of therapeutic options along with grade of evidence on their efficacy, to
enable an informed choice regarding treatment. It was recognized that the
choice of treatment in these cases would be dictated by the experience and
preference of the physician and the cost of therapy.
The Group underscored the need for randomized
controlled trials to compare the efficacy and safety of various treatment
regimens. In view of the clinical and histological heterogeneity of the
condition, these prospective trials must be appropriately stratified and
adequately powered to show clinically significant differences in outcome.
Studies comparing mycophenolate mofetil and dexa-methasone with
cyclosporin alone (www. fsgstrial.org; NCT001135811) and intravenous
cyclophosphamide with tacrolimus are underway. Further refinements and
standardization of care for patients with steroid resistant nephrotic
syndrome is likely to occur following results from these studies.
Writing Committee
Ashima Gulati, Arvind Bagga, Sanjeev Gulati, KP Mehta
and M Vijayakumar, on behalf of the Indian Society of Pediatric
Nephrology.
|
ANNEXURE–I
Members of the Review Committee
Kamran Afzal, Jawaharlal Nehru Medical College,
Aligarh; Indira Agarwal, Christian Medical College Hospital,
Vellore; Vinay Agarwal, Max Hospital, New Delhi; Uma Ali,
Bai Jerbai Wadia Hospital for Children, Mumbai; Sanjeev Bagai,
Rockland Hospital, New Delhi; Arvind Bagga, All India Institute
of Medical Sciences, New Delhi (Convenor); Sushmita
Banerjee, Calcutta Medical Research Institute, Kolkata; Ashima
Gulati, All India Institute of Medical Sciences, Delhi; Sanjeev
Gulati, Fortis Hospital, New Delhi; Pankaj Hari, All India
Institute of Medical Sciences, New Delhi (Secretary);
Arpana Iyengar, St. John’s Medical College, Bangalore; OP
Jaiswal, Sunder Lal Jain Hospital, New Delhi; Rupesh Jain,
Ekta Hospital for Children, Raipur; M Kanitkar, Armed Forces
Medical College, Pune; Mukta Mantan, Maulana Azad Medical
College, New Delhi; Kamini Mehta, Lilavati Hospital & Research
Center, Mumbai; Kumud Mehta, Jaslok Hospital & Research Center
& Bai Jerbai Wadia Hospital for Children, Mumbai; BR
Nammalwar, Kanchi Kamakoti CHILDS Trust Hospital, Chennai;
Amitava Pahari, Apollo Hospital, Kolkata; Saroj K Patnaik,
No.12 Air Force Hospital, Gorakhpur; KD Phadke, St. John’s
Medical College, Bangalore; N Prahlad, Mehta Children’s
Hospital, Chennai; PK Pruthi, Sir Gangaram Hospital, New Delhi;
Abhijeet Saha, Government Medical College, Chandigarh; VK
Sairam, Sri Ramchandra Medical College, Chennai; Jayati
Sengupta, AMRI Hospital, Kolkata; Prabha Senguttuvan,
Institute of Child Health, Chennai (Chairperson); Sidharth K Sethi,
All India Institute of Medical Sciences, New Delhi; Mehul Shah,
Apollo Hospital, Hyderabad; Jyoti Sharma, Bharti
Vidyapeeth Medical College, Poona; RN Srivastava, Indraprastha
Apollo Hospital, New Delhi; AS Vasudev, Indraprastha Apollo
Hospital, New Delhi; Anil Vasudevan, St. John’s Medical
College, Bangalore; and M Vijayakumar, Mehta Children’s
Hospital, Chennai.
|
Funding: None.
Competing interests: None stated.
References
1. Indian Pediatric Nephrology Group, Indian Academy of
Pediatrics. Management of steroid sensitive nephrotic syndrome: Revised
guidelines. Indian Pediatrics 2008; 45: 203-214.
2. Bagga A, Mantan M. Nephrotic syndrome in children.
Indian J Med Res 2005; 122: 13-28.
3. Rowe G, Wright G. The Delphi technique as a
forecasting tool: issues and analysis. Int J Forecasting 1999; 15:
353-375.
4. Ozen S, Ruperto N, Dillon MJ, Bagga A, Barron K,
Davin JC, et al. EULAR consensus criteria for classification of
childhood vasculitides. Ann Rheum Dis 2006; 65: 936-941.
5. Carruthers SG, Larochelle P, Haynes RB, Petrasovits
A, Schiffrin EL. Report of the Canadian Hypertension Society Consensus
Conference.1. Introduction. Can Med Assoc J 1993; 149: 289-293.
6. International Study of Kidney Disease in Children.
The primary nephrotic syndrome in children. Identification of patients
with minimal change nephrotic syndrome from initial response to
prednisone. J Pediatr 1981; 98: 561-564.
7. Habashi D, Hodson E, Craig J. Interventions for
idiopathic steroid-resistant nephrotic syndrome in children. Cochrane
Database System Rev 2007; 2: CD 003594.
8. Nammalwar BR, Vijayakumar M, Prahlad N. Experience
of renal biopsy in children with nephrotic syndrome. Pediatr Nephrol 2006;
21: 286-288.
9. International Study of Kidney Disease in Children.
Primary nephrotic syndrome in children: clinical significance of
histopathologic variants of minimal change and of diffuse mesangial
hypercellularity. Kidney Int 1981; 20: 765-771.
10. Fujinaga S, Kaneko K, Muto T, Ohtomo H, Murakami H,
Yamashiro Y. Independent risk factors for chronic cyclosporine induced
nephropathy in children with nephrotic syndrome. Arch Dis Child 2006; 91:
666-670.
11. Antignac C. Molecular basis of steroid resistant
nephrotic syndrome. Nefrologia 2005; 25 (suppl): 25-28.
12. Hinkes BG, Mucha B, Vlangos CN, Gbadegesin R, Liu
J, Hasselbacher K et al: Arbeitsgemeinschaft für Paediatrische
Nephrologie Study Group. Nephrotic syndrome in the first year of life: two
thirds of cases are caused by mutations in 4 genes (NPHS1, NPHS2, WT1, and
LAMB2). Pediatrics 2007; 119:e907-919.
13. Ruf RG, Lichtenberger A, Karle SM, Has JP, Anacleto
FE, Schultheiss M, et al. Patients with mutations in NPHS2
(podocin) do not respond to standard steroid treatment of nephrotic
syndrome. J Am Soc Nephrol 2004; 15: 722-732.
14. Orloff MS, Iyengar SK, Winkler CA, Goddard KA, Dart
RA, Ahuja TS, et al. Variants in the Wilms’ tumor gene are
associated with focal segmental glomerulosclerosis in the African American
population. Physiol Genomics 2005; 21: 212-221.
15. Schwaderer P, Knüppel T, Konrad M, Mehls O, Schärer
K, Schaefer F, et al. Clinical course and NPHS2 analysis in
patients with late steroid-resistant nephrotic syndrome. Pediatr Nephrol
2008; 23: 251-256.
16. Gulati S, Sengupta D, Sharma RK, Sharma A, Gupta
RK, Singh U, et al. Steroid resistant nephrotic syndrome. Indian
Pediatr 2006; 43: 55-60.
17. Ehrich JH, Geerlings C, Zivicnjak M, Franke D,
Geerlings H, Gellermann J. Steroid-resistant idiopathic childhood
nephrosis: overdiagnosed and undertreated. Nephrol Dial Transplant 2007;
22: 2183-2193.
18. Thomas DB, Franceschini N, Hogan SL, Holder S,
Jennette CE, Falk RJ, et al. Clinical and pathological
characteristics of focal segmental glomerulosclerosis pathologic variants.
Kidney Int 2006; 69: 920-926.
19. Collaborative Study of the Adult Idiopathic
Nephrotic Syndrome. A controlled study of short-term prednisone treatment
in adults with membranous nephropathy. N Engl J Med 1979; 301: 1301-1306.
20. Garin EH, Orak JK, Hiott KL, Sutherland SE.
Cyclosporine therapy for steroid resistant nephrotic syndrome. A
controlled study. Am J Dis Child 1988; 142: 985-988.
21. Lieberman KV, Tejani A. A randomized double-blind
placebo-controlled trial of cyclosporine in steroid resistant idiopathic
focal segmental glomerulosclerosis in children. J Am Soc Nephrol 1996; 7:
56-63.
22. Ponticelli C, Rizzoni G, Edefonti A, Altieri P,
Rivolta E, Rinaldi S, et al. A randomized trial of cyclosporine in
steroid resistant idiopathic nephrotic syndrome. Kidney Int 1993; 43:
1377-1384.
23. Niaudet P, Habib R. Cyclosporine in the treatment
of idiopathic nephrosis. J Am Soc Nephrol 1994; 4: 1049-1056.
24. Gulati S, Prasad N, Sharma RK, Kumar A, Gupta A,
Baburaj VP. Tacrolimus: a new therapy for steroid resistant nephrotic
syndrome in children. Nephrol Dial Transplant 2008; 23: 910-913.
25. Choudhry S, Bagga A, Menon S, Hari P. Randomized
controlled trial on efficacy and safety of cyclosporin vs
tacrolimus in steroid resistant nephrotic syndrome. Pediatr Nephrol 2007;
22: 1480 (abstract).
26. International Study of Kidney Disease in Children.
Prospective, controlled trial of cyclophosphamide therapy in children with
the nephrotic syndrome. Lancet 1974; ii: 423-427.
27. Tarshish P, Tobin JN, Bernstein J, Edelmann CM Jr.
Cyclophosphamide does not benefit patients with focal segmental
glomerulosclerosis. A report for the International Study of Kidney Disease
in Children. Pediatr Nephrol 1996; 10: 590-593.
28. Elhence R, Gulati S, Kher V, Gupta A, Sharma RK.
Intravenous pulse cyclophosphamide – a new regime for steroid resistant
minimal change nephrotic syndrome. Pediatr Nephrol 1994; 8: 1-3.
29. Bajpai A, Bagga A, Hari P, Dinda A, Srivastava RN.
Intravenous cyclophosphamide in steroid resistant nephrotic syndrome.
Pediatr Nephrol 2003; 18: 351-356.
30. Adhikari M, Bhimma R, Coovadia HM. Intensive pulse
therapies for focal glomerulosclerosis in South African children. Pediatr
Nephrol 1997; 11: 423-428.
31. Mendoza SA, Reznik VM, Griswold WR, Krensky AM,
Yorgin PD, Tune BM. Treatment of steroid resistant focal segmental
glomerulosclerosis with pulse methylprednisolone and alkylating agents.
Pediatr Nephrol 1990; 4: 303-307.
32. Hari P, Bagga A, Mantan M. Short term efficacy of
intravenous dexamethasone and methylpre-dnisolone therapy in steroid
resistant nephrotic syndrome. Indian Pediatr 2004; 41: 993-1000.
33. Mantan M, Sriram CS, Hari P, Dinda A, Bagga A.
Efficacy of intravenous pulse cyclophosphamide treatment versus
combination of intravenous dexamethasone and oral cyclophosphamide
treatment in steroid-resistant nephrotic syndrome. Pediatr Nephrol 2008;
23:1495-1502
34. Plank C, Kalb V, Hinkes B, Hildebrandt F, Gefeller
O, Rascher W, for Arbeitsgemeinschaft für Pädiatrische Nephrologie.
Cyclosporin A is superior to cyclophosphamide in children with
steroid-resistant nephrotic syndrome – a randomized controlled
multicentre trial by the Arbeitsgemeinschaft für Pädiatrische Nephrologie.
Pediatr Nephrol 2008; 23:1483-1493.
35. Rao J, Xu H, Cao Q, Huang WY, Zhou LJ. Comparison
of cyclophosphamide and cyclosporine in the treatment of steroid-resistant
idiopathic nephrotic syndrome in children. Zhong Nan Da Xue Bao Yi Xue Ban
2007; 32: 958-963.
36. Heng Li, Xiayu Li, Jianghua Chen. Tacrolimus versus
pulse intravenous cyclophosphamide therapy in adults with
steroid-resistant idiopathic minimal change nephropathy: A prospective
case-matched trial in china. J Am Soc Nephrol 2008; 19: 557A
37. Choi MJ, Eustace JA, Gimenez LF, Atta MG, Scheel
PJ, Sothinathan R, et al. Mycophenolate mofetil treatment for
primary glomerular diseases. Kidney Int 2002; 61: 1098-1114.
38. Bosch T, Wendler T. Extracorporeal plasma treatment
in primary and recurrent focal segmental glomerular sclerosis. Ther Apher
2001; 5: 155-160.
39. Bagga A, Sinha A, Moudgil A. Rituximab in patients
with the steroid resistant nephrotic syndrome. N Engl J Med 2007; 356:
2751-2752.
40. Cattran DC, Alexopoulos EH, Heering P, Hoyer PF,
Johnston A, Meyrier A, et al. Cyclosporin in idiopathic glomerular
disease associated with the nephrotic syndrome: Workshop recommendations.
Kidney Int 2007; 72: 1429-1447.
41. Troyanov S, Wall CA, Mille JA, Scholey JW, Cattran
DC. Focal and segmental glomerulosclerosis: definition and relevance of a
partial remission. J Am Soc Nephrol 2005; 16: 1061-1068.
42. Goumenos DS, Kalliakmani P, Tsakas S, Savidaki I,
Vlachojannis JG. Cyclosporin A in the treatment of nephrotic syndrome: the
importance of monitoring C0 (trough) and C2 (two hours after
administration) blood levels. Med Chem 2006; 2: 391-393.
43. Ahmad H, Tejani A. Predictive value of repeat renal
biopsies in children with nephrotic syndrome. Nephron 2000; 84: 342-346.
44. Yi Z, Li Z, Wu XC, He ON, Dang XO, He XJ. Effect of
fosinopril in children with steroid-resistant idiopathic nephrotic
syndrome. Pediatr Nephrol 2006; 21: 967-972.
45. Bagga A, Mudigoudar BD, Hari P, Vasudev V.
Enalapril dosage in steroid-resistant nephrotic syndrome. Pediatr Nephrol
2004; 19: 45-50.
46. Kunz R, Friedrich C, Wolbers M, Mann JF.
Meta-analysis: effect of monotherapy and combination therapy with
inhibitors of the renin angiotensin system on proteinuria in renal
disease. Ann Intern Med 2008; 148: 30-48.
47. Clinical practice guidelines for managing
dyslipidemias in kidney transplant patients: a report from the Managing
Dyslipidemias in Chronic Kidney Disease Work Group of the National Kidney
Foundation Kidney Disease Outcomes Quality Initiative. Am J Transplant
2004; 4 (Suppl 7): 13-53.
48. Prescott WA, Streetman DA, Streetman DS. The
potential role of HMG-CoA reductase inhibitors in pediatric nephrotic
syndrome. Ann Pharmacother 2004; 38: 2105-2114.
|
|
|
 |
|

