This study was undertaken from April 2002 to
March 2003 to find out the correlation of transcutaneous
bilirubinometer index with serum bilirubin levels in term,
pre-term, small for gestation age babies, with and without
phototherapy in neonates with jaundice. Another aim was to
evaluate the transcutaneous bilirubinometer as a screening
device for neonatal hyperbilirubinemia by finding the action
levels for TcBI at forehead and sternum at which sample for
serum bilirubin estimation should be taken. A total of 104
neonates were evaluated. Mean (SD) age (hours), birth weight
(grams) and gestational age (weeks) were 100.4 (37.90), 2264.9
(634.4) and 36.8 (2.9) respectively. Mean serum bilirubin was
16.6 (6) mg/dL. Overall a correlation coefficient of 0.878 at
forehead and 0.859 at sternum was observed. On excluding infants
receiving phototherapy coefficients of 0.900 at forehead and
0.908 at sternum were noted. Correlation coefficient over
forehead and sternum was found to drop from 0.85 to as low as
0.33 with duration of phototherapy exceeding 48 hrs. Lastly the
determined action levels had a sensitivity of 77.8 to 100 % in
assessing the need for serum bilirubin estimation in various
groups.
Key words: Hyperbilirubinemia, Neonatal jaundice,
Transcutneous bilirubinometery.
Transcutaneous bilirubinometry measures the
intensity of yellow color in the skin and subcutaneous tissue and
correlates it with the serum bilirubin concentration in newborn
infants(1). It is reported to be safe, simple, objective,
reproducible, cost effective, noninvasive modality in the
screening and monitoring of jaundiced newborn infants. A
significant correlation has been found between serum bilirubin and
TcB index (TcBI) in various ethnic groups by various authors. But
the correlation is affected by gestational age, use of
phototherapy, birth weight, color of skin, degree of jaundice and
race(2-6).
Previous studies of this region either have
comparatively limited number of observations or lack mention of
range of bilirubin observed(5,6). Moreover, considering variations
in reported intercept in regression lines for different races(3),
there is a need for separate regression equation for different
regions. We evaluated transcutaneous bilirubin (TcB) over a wide
range of bilirubin, to find out the correlation of TcBI with serum
bilirubin levels in term, pre-term, small for gestation age
babies, with and without phototherapy in neonates with jaundice in
Shimla region. Another aim was to evaluate the TcB as a screening
device for neonatal hyperbilirubinemia by finding the action
levels for TcBI at forehead and sternum at which sample for serum
bilirubin estimation should be taken.
Subjects and Methods
The present study was undertaken at our centre
from 1st April 2002 to 31st March 2003. The study group included
both inborn and out born term and preterm neonates developing
clinical jaundice within first 10 days of life. Babies with major
congenital malformations, blood group group incompatibility,
conjugated hyperbilirubinemia, post exchange transfusion and those
dying during the study were excluded from the study group. Serum
bilirubin estimation and TcBI readings were taken as soon as a
baby with jaundice was admitted or as soon as jaundice appeared in
an already hospitalized baby. Thereafter, daily serum bilirubin
estimation and TcBI readings were taken in each case till jaundice
subsided. TcBI reading and bilirubin estimation was done by
different observers who were unaware of each others results and
the instruments were standardized according to the manufacturer’s
guidelines.
Detailed history and examination of babies
enrolled in the study was carried out and provisional clinical
diagnosis was assigned. After obtaining informed consent from
parents blood samples were drawn from a peripheral vein. Blood
samples for serum bilirubin estimation were taken into heparinized
capillaries and were placed in labeled dark colored tubes and kept
in light proof box until the moment of bilirubin determination.
Blood samples were taken within half hour of taking TcBI and were
analyzed by Wako Bilirubin tester Model SE 101 D II. Using the
Minolta Air-shields Jaundicemeter 101 two readings at forehead
just above the glabella and two at mid sternum were taken in a
quite child and mean of each was recorded. Eyes were shielded
while taking the readings at forehead and the probe was
disinfected with 70% isopropyl alcohol after using it on each
baby.
Clinical assessment of jaundice was done in
each baby at least twice a day in diffuse natural light. When
indicated phototherapy was given using Atom Phototherapy unit
PIT-220ST which was stopped while taking TcBI reading.
For analysis the study group was divided into
the following groups: term AFD (appropriate for date); term
SFD(small for date); preterm AFD; and preterm SFD
In each of the above groups, subgroup analysis
was done based on exposure to phototherapy.
In each of the above mentioned groups Pearson’s
correlation analyses and linear regression analyses were performed
using the SPSS statistical software. Usefulness of TcBI as a
diagnostic tool was evaluated by sensitivity, specificity,
positive predictive value and negative predictive values as per
standard methodology.
Results
A total of 104 neonates with grossly uniform
skin color were evaluated .The mean age in hours was 100.4 (37.9)
(SD) range (36 -200), mean birth weight in grams 2264.9 ± 634.4
(SD) range (1000-3600) and gestational age varied from 28 to 42
weeks with mean of 36.8 ± 2.9 (SD) weeks. The mean serum bilirubin
in mg/dL was 16.6 ± 6.1 (SD) range (7.0-34.0), the mean TcBI
reading over forehead and sternum was 22.7 ± 4.99 (SD) range
(14.0-37.0) and 21.2 ± 4.9 (SD) range (12.0-36) respectively.
The correlation co-efficient ranged from
0.468-0.954 for the various groups (Table I) and all but
one (Preterm SFD with phototherapy) were statistically significant
(P <0.05).
|
Table I
Correlation of TcBI and Total Serum Bilirubin in Various
groups |
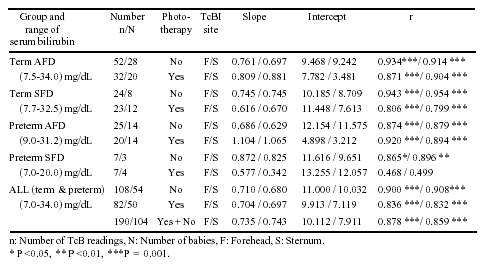 |
In babies who received phototherapy for a
duration of 12 to 48 hrs, correlation coefficient ranged from
0.829 to 0.857 (P <0.001) at the forehead and 0.793 to 0.839 (P
<0.001) at sternum. Those who received phototherapy for more than
48 hr, poor correlation coefficient of 0.329 (P >0.05) was found
at the forehead and a fair correlation coefficient of 0.539(P
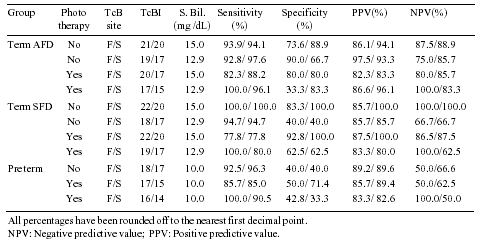
<0.05) was found at sternum. Table II depicts the action
levels determined for our infant population.
|
Table II
Suggested Action Levels for Different Groups
in the Present Study
|
 |
Discussion
If every visibly jaundiced neonate had serum
bilirubin estimation done, unnecessary skin puncture would be
performed on close to half of them. If only jaundiced neonates at
risk for bilirubin encephalopathy were sampled, a large number of
unnecessary skin puncture could be avoided. The use of TcB reading
as a screening device for neonatal hyperbili-rubinemia is based on
the assumption that serum and tissue bilirubin are in constant
equilibrium. Skin pigmentation has a profound effect on TcBI and
this has been illustrated by marked differences of the ‘y’-
intercept in the regression formula for the various racial groups.
The darker is the skin, the greater the ‘y’- intercept. In the
present study, Minolta Air-shields Jaundicemeter 101 was evaluated
in jaundiced babies during first 10 days of life and a good
correlation was found between total serum bilirubin level and TcBI
over forehead and sternum in almost all groups (Table II).
Further, action levels for TcBI at forehead and sternum were found
at which sample for serum bilirubin estimation could be taken.
Our data reveals a good correlation coefficient
of 0.878 at forehead and 0.859 at sternum and was comparable to
results of other workers(4,8). On excluding infants receiving
phototherapy we found an increase in correlation coefficient to
0.900 at forehead and 0.908 at sternum. This was better than that
found by Bhat, et al.(9) (r = 0.70) and Guha, et al.(7)
(r = 0.84).On evaluating infants who received phototherapy, the
coefficient of 0.836 at forehead and 0.832 at sternum was
comparable to that found by Aroor, et al.(4).
For all the above groups slope and intercept of
the regression line in the present study were different from those
of other studies. This is because of the laboratory variation in
serum bilirubin estimation, variation in TcB instrument and ethnic
differences in the basal yellowness of the skin, the more the
confounding variables present the poorer the correlation.
In our study, as the duration of phototherapy
increased correlation coefficient over forehead and sternum was
found to drop from 0.85 to as low as 0.33 with duration exceeding
48 hr. For infants undergoing phototherapy this relationship
between TcB and serum bilirubin level appears to be disrupted as
bleaching as well as tanning of the skin may affect the accuracy
of the TcB and the TcBI will be lower than expected for the same
serum bilirubin value.
The action levels determined for our population
correctly assessed the need for serum bilirubin estimation in most
cases. For neonatal hyperbilirubinemia a screening procedure
should have a high sensitivity and low rate of false negative
observations , so that no neonate with the cut off level is missed
by the procedure even if a few cases are falsely picked up . In
our study a small number of cases were missed by the action levels
we determined but the levels studied by us do not pose serious
danger to a healthy newborn. We agree with other workers that it
should be used for screening and not as an alternative to serum
bilirubin estimation(10,11).
In the present study, specificity was low in
some groups due to the fact that very few babies with low serum
bilirubin levels were included in the study group. If the babies
with low serum bilirubin levels had been included in the study
group, specificity would have increased. Cut off levels of TcBI
for action levels at various serum bilirubin could not be compared
with those found by other authors, since these are different for
different institutions. Small number of preterms in our study
preclude any meaningful assessment in this subgroup and larger
studies in preterms of various maturity and nutritional states are
needed. Studies are also required to evaluate this device for
screening jaundiced neonates in primary health care setting in
regions with homogenous ethinic background.
Contributions: The concept and design of
the study was provided by RKK and RLS which was carried out by GM
and MN. GM & NS analyzed the data, drafted the manuscript which
was finally revised and approved by RKK. RKK will act as the
guarantor.
Funding: None.
Competing Interests: None.