Sudha Rao, M.P. Colaco, M.P. Desai
From Bai Jerbai Wadia Hospital for Children
& Institute of Child Health and Research. Acharya Dhonde
Marg, Parel, Mumbai, India.
Correspondence to: Dr. Sudha Rao, Lecturer,
Division of Pediatric Endocrinology, Department of Pediatrics,
Bai Jerbai Wadia Hospital for Children, Acharya Dhonde Marg,
Parel, Mumbai 400 012. E-mail:
[email protected]
Manuscript received: June 26, 2001; Initial
review completed: August 7, 2001;
Revision accepted: August 7, 2002.
McCune Albright Syndrome (MCAS) is an
association of, Café-au-lait macules, polyostotic fibrous
dysplasia and autonomous hyperfunctioning endocrinopathy. This
is a rare disorder seen more commonly in females. We evaluated 7
(6F & 1M) cases under six years of age (4 months to 5.5 yrs)
presenting with Café-au-lait spots, polyostotic fibrous
dysplasia and/or sexual precocity. All the 7 cases had large
Café-au-lait spots, radiologic features of polyostotic fibrous
dysplasia were seen in 5 cases. Six girls had precocious puberty
with large ovarian follicles and elevated S. Estradiol levels
(14-65 pg/dl) with prepubertal gonadotropin levels in 5 of them.
Medroxy-progrestrone acetate was used to treat the sexual
precocity. Five girls on follow up for 6 months (6mo-16mo)
showed cessation of menstrual episodes and regression of ovarian
follicles in three, regression in breast size in one, and three
girls continued to grow at a height velocity >95th centile
for age. Skeletal lesions and skin features did not show any
change. No other endocrinopathy was noted. Gonadotropin
independent precocious puberty was the only endocrine affection
seen in this series.
Key words:
McCune-Albright syndrome, Café-au-lait
spots, Polyostotic fibrous dysplasia.
McCune Albright Syndrome (MCAS) is a
heterogeneous clinical condition(1) which is caused by a sporadic,
somatic, post zygotic missens mutation in the gene codifying the
alpha subunit of Gs protein of the receptor system of most proteic
hormones(2). This is a rare syndrome, seen more commonly in
females. The classic form is defined by a triad of physical signs:
melanotic cutaneous macules called café-au-lait spots,
multi-centered but localized osseous lesions termed polyostotic
fibrous dysplasia and autonomous hyperfunctioning
endocrinopathies(1). The non-classical form consists of only two
of these conditions(3,4). Gonadotropin indepen-dent precocious
puberty is the commonest endocrine affection described(1,6). Until
now, there are no reports of a series of cases of MCAS in Indian
literature. We describe here 7 cases of McCune Albright Syndrome (MCAS)
referred to the Endocrinology Division of this institution along
with a review of litetrature.
Subjects and Methods
Seven children (6F, 1M) under the age of six
years (4mo - 5.5yrs) diagnosed to have MCAS on the basis of the
presence of café-au-lait spots, radiological evidence of
polyostotic fibrous dysplasia, and/or sexual precocity were
studied. The presence of typical, irregularly shaped
hyperpigmented café-au-lait spots; their number, size, shapes and
localization were recorded. The presentation with the development
of secondary sexual characteristics prior to the age of 8 years in
girls and 9 years in boys was suggestive of sexual precocity. The
pubertal signs were assessed based on Tanner’s staging. Skeletal
dysplasia recognised at clinical level (pain, gait disturbance,
fractures, and deformities) was graded using the Feullian’s
score(5). Other associated endocrine and non-endocrine findings
were also noted. All the cases were subjected to laboratory
investigations. Serum calcium, phosphorous and alkaline
phosphatase levels were done in all the cases. Serum FSH, LH and
Estradiol / Testosterone levels were done in the cases with
clinical evidence of precocious puberty. Specific endocrine
investigations viz. T3, T4, TSH, Prolactin levels were done as
indicated. Pelvic sonography was done in all girls to assess the
uterine size, check for ovarian cysts. Radiography of the limb
bones, pelvis and skull was done in all the cases to detect
monostotic or polyostotic fibrous dysplasia. Bone scintigraphy
could not be done in the children who did not show any
radiological changes. Special investigations in the form of
echocardiography were done in cases presenting cardiac affection.
Medroxy-progesterone acetate (MPA) was given, in
a dose of 150mg, intramuscularly once every 4 weeks in all the
girls presenting with sexual precocity and episodes of vaginal
bleeding. Periodic follow up at 3 months interval included a
detailed clinical evaluation of the progress of puberty, menstrual
episodes and growth velocity. Repeat pelvic ultrasound and
estimation of estradiol levels was advised in the females.
Results
The age of onset of symptoms ranged between 3mo
to 4yr 6mo. Sexual precocity was the commonest endocrine affection
seen. Five patients had the characteristic triad of café-au-lait
spots, polyostotic fibrous dysplasia and precocious puberty; two
cases (case 1 & 4) presented in the non-classical form (Table
I). Out of all the six girls who presented with sexual
precocity, five had episodes of bleeding per vaginum (Table I).
The vaginal mucosa appeared pale with presence of white discharge
in all these 6 cases. The café-au-lait spots, seen in all the 7
cases, were restricted to one half of the body (Fig 1).
Skeletal involvement was moderate to severe in four cases (Table
I). Goiter in two cases (case 1&4) and soft systolic murmur in
two cases (case 2 & 5) were the other associated findings.
Laboratory investigations (Table I) showed elevated S.
Estradiol levels (14-65 pg/dL) in all the 6 girls. Pubertal
gonadotropin levels (FSH: 5.2mIU/ml, LH: 7.6IU/ml) was seen in one
child (case-4), possibly due to the onset of secondary true
precocious puberty. Thyroid profile done in 4 cases was normal.
Alkaline phosphatase was uniformly raised, markedly so in the
cases with severe skeletal dysplasia.
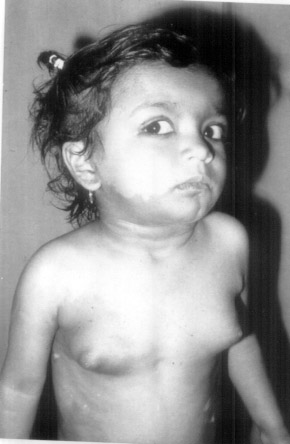 |
| Fig.
1 Showing the Cafe-au-lait spotts on right side of the face,
neck and trunk. Also note the thelarche (Tanner's stage III
of breast development). |
Table I__Case Description
| Case |
Age of
Presentation |
Age of onset
symptoms (B-H-T)* |
Sex |
Vaginal bleeding & cycles |
$ SMR (Tanner's stage) |
Cafe-au-laits |
Bony Deformity/ bone pain (feullian
score) |
Estradiol pg/gl (N<10) |
ultrasonography O/Ass. |
X-ray Features |
1
|
3 yrs 10 mo
|
3 yrs 3 mo
|
M
|
–
|
G1P1A1
|
multiple largest
|
+ genu valgum,
|
–
|
–
|
generalised
|
|
|
|
|
|
(I)
|
5cm × 20 cm
|
# Rt femur,
|
|
|
osteopenia, cortical
|
|
|
|
|
|
|
left leg, buttock,
|
# Left tibia
|
|
|
thinning, extensive
|
|
|
|
|
|
|
flank, upper
|
(Severe)
|
|
|
base of skull sclerosis
|
|
|
|
|
|
|
back
|
|
|
|
B/L healed # neck Femur
|
2
|
2yrs 10 mo
|
2yrs-T
|
F
|
Regular
|
B3P2A0
|
several all over
|
+genu valgum, hip
|
14.0
|
Uterus=4.3×
|
#Rt femur Sclerosis
|
|
|
2yr6mo-B
|
|
|
(III)
|
the body
|
dislocation,
|
|
2.3×1.5
|
base skull fibrous
|
|
|
2yr 6mo-H
|
|
|
|
|
beading
|
|
N endo.echo
|
dysplasia long bone leg
|
|
|
|
|
|
|
|
(Moderate)
|
|
Multiple cysts
|
|
|
|
|
|
|
|
|
|
in ovaries RtOv=
|
|
|
|
|
|
|
|
|
|
large cyst 4.3×3.5
|
|
|
|
|
|
|
|
|
|
×3cm LtOv=small
|
|
|
|
|
|
|
|
|
|
cyst 1.7×0.7cm
|
3
|
5yr 6mon
|
1yr-T
|
F
|
Irregular
|
B2P1A1
|
Large over
|
+genu valgum,
|
48.0
|
Ut=4.4×2.8
|
Fibrous dysplasia rt
|
|
|
1yr6mo-B
|
|
|
(II)
|
spine and
|
#Rt femur
|
|
×1.8 Endo
|
femur
|
|
|
|
|
|
|
buttocks
|
(Severe)
|
|
thick=0.6 Lt.Ov
|
|
|
|
|
|
|
|
|
|
large cyst 3.6×
|
|
|
|
|
|
|
|
|
|
3.6×2.1 Rt.Ov=
|
|
|
|
|
|
|
|
|
|
1.2×1.4×0.6
|
|
|
|
|
|
|
|
|
|
two follicls Rt Ov
|
4.
|
4yrs 11mo
|
4y7mo-B
|
F
|
Once
|
B3P1A1
|
Irregular 5×4 cm
|
-beading+
|
65.2
|
Ut=4.2×3×1.2 Rt
|
Rachitic changes+
|
|
|
4yr6mo-T
|
|
|
(III)
|
over
|
|
|
Ov=2×1.5×2
|
|
|
|
|
|
|
sacrum.Also
|
(NIL)
|
|
LtOv=not visualised
|
|
|
|
|
|
|
over inguinal
|
|
|
i.e. small follicles
|
|
|
|
|
|
|
region
|
|
|
both sides
|
5.
|
2Yr 8mo
|
3mo-T
|
F
|
–
|
B4P1A1
|
+over face Rt
|
+ pain
|
32.0
|
Ut=4×1 bulky
|
fibrous dysplasia over
|
|
|
|
|
|
(IV)
|
trunk
|
(MILD)
|
|
LtOv=1.7×1, Rt OV
|
rt fibula-lower end
|
|
|
|
|
|
|
|
|
|
not visualised
|
6.
|
4 Mo
|
3mo-B
|
F
|
Once
|
B2P1A1
|
large over
|
(NIL)
|
22.0
|
i.e. follicles bulky ut.
|
–
|
|
|
|
|
|
(II)
|
buttocks rt back
|
|
|
RtOv-2.2×1.5×1.7
|
|
|
|
|
|
|
and arm
|
|
|
single large follicle 1cm
|
|
|
|
|
|
|
|
|
|
dia LtOv-1.2×1.1
|
7.
|
2yr8mo
|
1yr6mo-B
|
F
|
Irregular
|
B2-3P1A1
|
large on rt face
|
hemiatrophy waddling
|
52.0
|
Ut=4.9×1.6×2.0
|
fibrous
|
|
|
1yr6mo-T
|
|
|
(II)
|
8×4cm
|
gait genu valgum
|
|
Rt Ov-not seen
|
dysplasia hip
|
|
|
|
|
|
|
|
bone pain+ (Moderate)
|
|
Lt Ov-2.5×1.6 with
|
bones and
|
|
|
|
|
|
|
|
|
|
single follicle=2.4×1.4
|
neck Rt femur
|
|
|
|
|
|
|
|
*B-Bleeding per vaginum, T-Thelarche, H-public hair, $ SMR-Sexual maturity rating, Ov= Ovary
Pelvic ultrasound in all the girls showed bulky
uterus and asymmetric ovarian enlargement with large follicular
cysts on presentation (Table I). On radiography polyostotic
fibrous dysplasia was seen in 5 cases (Table I) (Fig 2).
Bone scintigraphy could not be done in the two cases (Cases 4
& 6) that did not show the radiological evidence.
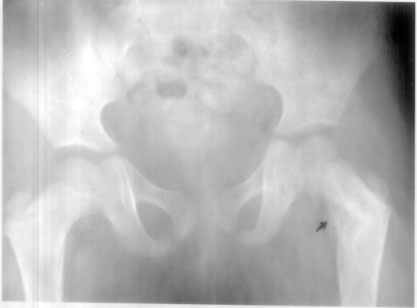 |
| Fig. 2 Radiological
evidence of fibrous dysplasia seen in the neck of left
femur. |
Five girls received monthly injections of
Medroxy-progestrone acetate (MPA) 150 mg. Other drugs like
testolactone were not available and/or affordable. All the five
girls could be followed up for a period of at least 6 months. A
height velocity >95th centile was seen in three cases,
(Case-3,4,7), Menstrual episodes did seem to respond in three
cases (Case-4,6,7). The breast size remained the same in three
(Case-2,3,7), regressed in one (Case-6) and advanced further in
one case (Case-4). Appearance of pubic hair was seen in one case.
Follow up investigations revealed a fall in repeat S. Estradiol
level in three cases (Case-2,6,7), it could not be done in the
other two. On the pelvic ultrasound, the uterine dimensions had
regressed in 3 cases (Cases 4,6,7). The period of follow up was
too short to judge any deterioration in the skeletal affection or
to observe any further skin changes. No other endocrine or
non-endocrine conditions were seen on follow up.
Discussion
McCune and Albright (1936) first described the
association of café-au-lait pigmented skin lesions and
polyostotic fibrous dysplasia with gonadotropin independent
precocious puberty. This is a rare disorder seen commonly in
females (1,5,6). Out of the 196 cases of precocious puberty seen
at our clinic in the last two decades, there were 7 cases that
presented as MCAS. MCAS is a sporadic condition caused by a
somatic, constitutively activating mutation in the exon 8 of Gs
alpha gene codifying the alpha subunit of Gs protein(2,3). This
abnormal Gs protein activates the adenylate cyclase system leading
to autonomous function of cell proliferation and/or hormonal
hypersecretion(6). The clinical expression depends on the number
of mutated cells and the affected organs; thus the presentation
can be heterogeneous. It can be of early or late onset with slow
or quick evolution(2).
Precocious puberty, which is gonadotropin
independent (prepubertal gonadotropin levels), is the commonest
endocrine affection and occurs due to the autonomous functioning
of ovarian cysts causing cyclic estrogen secretion which affects a
rapid progression of pubertal characteristics along with
accelerated growth and skeletal maturation(7). Breast enlargement
and pigmentation occurs with the development of follicular cyst
and uterine bleeding occurs with their involution(2,4,7). High
circulating estrogen levels increase the sensitivity of androgen
responsive organs and can cause growth of genital hair and apocrine sweat gland development despite prepubertal androgen
levels(7).
Autonomous hyperfunctioning of other endocrine
organs causing hyperthyrodism, occult thyrotoxicosis,
hypercortisolism, GH excess, hyperprolactinemia, are described.
Thyroid disorder is the second commonest affection seen(3,9). Bone
dysplasia, thymic hyperplasia, hepatobiliay disorders, intestinal
adenomatous polyp, cardiovascular disorders are some of the
non-endocrine manifestations(6,7). Bone dysplasia, can be mono-ostotic
or polyostotic. They mostly involve the limb bones. Clinically
they present with chronic pain, deformity, limb asymmetry and
spontaneous fractures. Distortion of facial features, auditory and
visual impairment; due to compression of acoustic and optic nerves
respectively can result from fibrous dysplasia of facial bones(8).
Plain radiographs may be normal early in life and a technetium
bone scan may be required(7).
Café-au-lait macules are large pigmented spots
with irregular borders (coast of Maine). They are of different
size, number, morphology, age of appearance, usually affecting one
half of the body and may indicate active melanocyte
proliferation(2). These skin changes may evolve over a period of
time.
As the precocious puberty is gonadotropin
independent, GnRH analogues have not been found to be useful in
the treatment(1). Medroxy-progesterone acetate (MPA) can be used
for its local anti-estrogen property. There are not many reports
on treatment with MPA in the literature(11). In our series, out of
the 5 cases that received MPA, three did not show any progression
of pubertal changes. Testolactone, an aromatase inhibitor, in a
dose of 40mg/kg/D in divided doses, has recently been shown to be
effective in causing a fall in estradiol levels, reduction in
menstrual episodes and growth velocity. Adverse effects in the
form of abdominal pain, headache, and abnormal liver enzymes are
reported(6). Tamoxifene, a nonsteroidal estrogen-antiestrogen,
which acts by competitive inhibition at the estrogen receptor
level, is used in a dose of 20-40mg/day. Experience of its use is
limited(12). Oophorectomy or cyst removal has sometimes been
helpful in a rapidly progressing pubertal development(8).
Bisphosphonates have been used in the treatment of moderate to
severe polyostotic fibrous dysplasia with variable response(13).
Other endocrine manifestations need specific treatment.
Acknowledgement
The authors wish to thank the patients and The
Management, Wadia Group of Hospitals for all the help rendered in
publishing this work.
Contributors:
SR collected, analyzed,
drafted the manuscript and will act as the guarantor. MPC and MPD
reviewed the subject and helped in analysis and drafting the
paper.
Funding: None.
Competing interests: None stated.
|
Key Messages |
-
McCune-Albright
Syndrome is a heterogeneous clinical conditon defined by a
triad of physical signs: melanotic cutaneous macules called
café-au-lait spots, mutli-centered but localized osseous
lesions termed polyostotic fibrous dysplasia and autonomous
hyperfunctioning endocrinopathies.
-
Gonadotropin
independent precocious puberty is the commonest endocrine
affection seen.
- About 10% of normal children
have café-au-lait spots but when they are associated with
autonomous endocrine hyperfunctioning and/or signs of cell
proliferation, MCAS must be suspected.
|
1. McCune DJ.
Osteitis fibrosa cystica; the case of a nine year old girl who
also exhibits precocious puberty, multiple hyperpigmentation of
the skin and hyperthyroidism. Am J Dis Child 1936; 52: 743-744.
2. de Sanctis C,
Lala R, Matarazzo P, Balsamo A, Bergmaschi R, Cappa M, et al.
McCune-Albright syndrome: a longitudinal follow up of 32
patients. J Pediatr Endocrinol Metab 1999; 12: 817-826.
3. Weinstein LS,
Shenker A, Gejman PV, Merino MJ, Friedman E, Spiegel AM.
Activating mutations of the stimulatory G protein in the McCune
– Albright syndrome. N Engl J Med 1991; 325: 1688-1695.
4. Feullian PP,
Johns J, Cutler GB. Longterm testalactone therapy for precocious
puberty in girls with McCune-Albright syndrome. J Clin
Endocrinol Metab 1993; 77: 647-651.
5. Albright F,
Butler AM, Hampton AO, Smith P. Syndrome characterized by
osteitis fibrosa disseminata, areas of pigmentation and
endocrine dysfunction with precocious puberty in females. N Engl
J Med 1937; 216:727-746.
6. Shenkar A,
Weinstein LS, Moran A, Pescovitz OH, Charest NJ, Boney Cm, et
al. Severe endocrine and non-endocrine manifestations of
the McCune-Albright syndrome associated with activating
mutations of stimulatory G protein Gs. J Pediatr 1993; 123:
509-518.
7. Low LCK, Wang
Q. Gonadotropin independent precocious puberty. J Pediatr
Endocrinol Metab 1998; 11: 497-507.
8. Nager GT,
Dennedy DW, Kopstein E. Fibrous dysplasia: a review of the
disease and its manifestations in temporal bone. Ann Otol Rhinol
Laringol 1982: 92(Suppl): 1-52.
9. Lee PA, Van
Dop C, Migeon CJ. McCune-Albright syndrome. Longterm follow up.
JAMA 1986; 256: 2980-2984.
10. Foster CM,
Comite P, Pescovitz OH, Ross JL, Loriaux DL, Cutler Gb Jr.
Variable responses to a long acting agoinst of leutinizing
hormone- releasing hormone in girls with McCune-Albright
syndrome. J Clin Endocrinol Metab 1984; 59: 801-805.
11. Barberi RL,
Ryan KT. Direct effects of medroxy-progestrone acetate (MPA) on
rat testicular steroidogenesis. Acta Endocrinol (Copenh) 1980;
94: 419-425.
12. Eugaer EA,
Shankar R, Feezle LK, Pescovitz OH. Tamoxifene treatment of
progressive precocious puberty in a patient with McCune-Albright
syndrome. J Pediatr Endocrinol Metab 1999; 12: 681-686.
13. Zacharin M, O’Sullivan M.
Intravenous pamidronate treatment of polyostotic fibrous
dysplasia associated with the McCune Albright syndrome J Pediatr
2000; 137: 403-409.
|
