|
Anaita Vdwadia-Hegde
Shashank Parekji
Uma S. Ali
Kumud P. Mehta
From the Department of Nephrology, Bai Jerbai
Wadia .
Hospital for Children, Parel,
Mumbai 400 012, india.
Reprint requests: Dr. Uma S. Ali, Head of Nephrology, Bai Jerbai Wadia Hospital for Children, Parel, Mumbai 400
012, india.
Manuscript Received: October 9, i997; initial review completed: December 10, 1997;
Revision Accepted: July 31, 1998
Angiotensin converting enzyme (ACE) inhibitors have gained widespread acceptance in the management of hypertension(1), since their discovery in 1965.. Their success is based largely on their limited side effects and the lack of metabolic changes. ACE inhibitors decrease vascular resistance, improve glucose handling, control left ventricular mass and produce little change in heart rate and cardiac output(1). The fact that they prevent the progress of nephropathy associated with both type I and II diabetes, makes it most likely that a large number of women of child bearing age group could be placed on this medication.
It was as early as 1980 that the first animal studies reported fetal wastage in ACE inhibitor exposed experiments. The first report of an adverse outcome in a human pregnancy was reported in 1982(2), and in
1985 warnings regarding the adverse fetal effects of ACE inhibitors
started appearing in literature. The FDA insisted on a box warning for all ACE inhibitors as late as 1992, stating that when used in the second and third trimesters of pregnancy, they could cause damage and even cause death of the fetus. There is no proof of first trimester toxicity till date, however one case of encephalocoel with first trimester exposure has been reported(3).
There are about 59 cases of ACE inhibitor fetopathy in literature till date(4). Ours is the first case to be reported in Indian literature.
Case Report
A 35-year-old woman o! a third degree consanguineous marriage conceived for the seventh time. She had 4 children, all females, alive and well. After the fourth pregnancy she developed chronic hypertension and was put on antihypertensives namely enalapril, alpha-methyldopa, atenolol and nifidepine. During the next few years she had two spontaneous abortions at five and four months of amenorrhea, respectively. She had been on antithypertensive medication for three years prior to her seventh conception. During her pregnancy she was diagnosed by ultrasound to have oligohydramnios at seven months of amenorrhea. This oligohydramnios was
progressive and the mother was taken up for elective LSCS at 34 weeks gestation.
The child was a 1.70 kg male preterm. He was refered to us on day 6 of life for anuria since birth as noted by the mother and the attending doctor. On examination the child had marked sutural separation with
hypocalvaria, left simian
crease, bilateral lower limb edema, abnormal right auricle and
overriding of fourth and fifth toes. Kidneys and bladder were not palpable. Neurologically the child was markedly depressed and biochemical parameters were suggestive of acute renal failure.
Continuous non-invasive monitoring (NIBP) showed a systolic blood pressure of 70 mm Hg. Ultrasonography showed normal sized kidneys. Color Doppler showed normal arterial and venous blood flow. X-ray skull showed evidence of hypocalvaria (Fig.1).
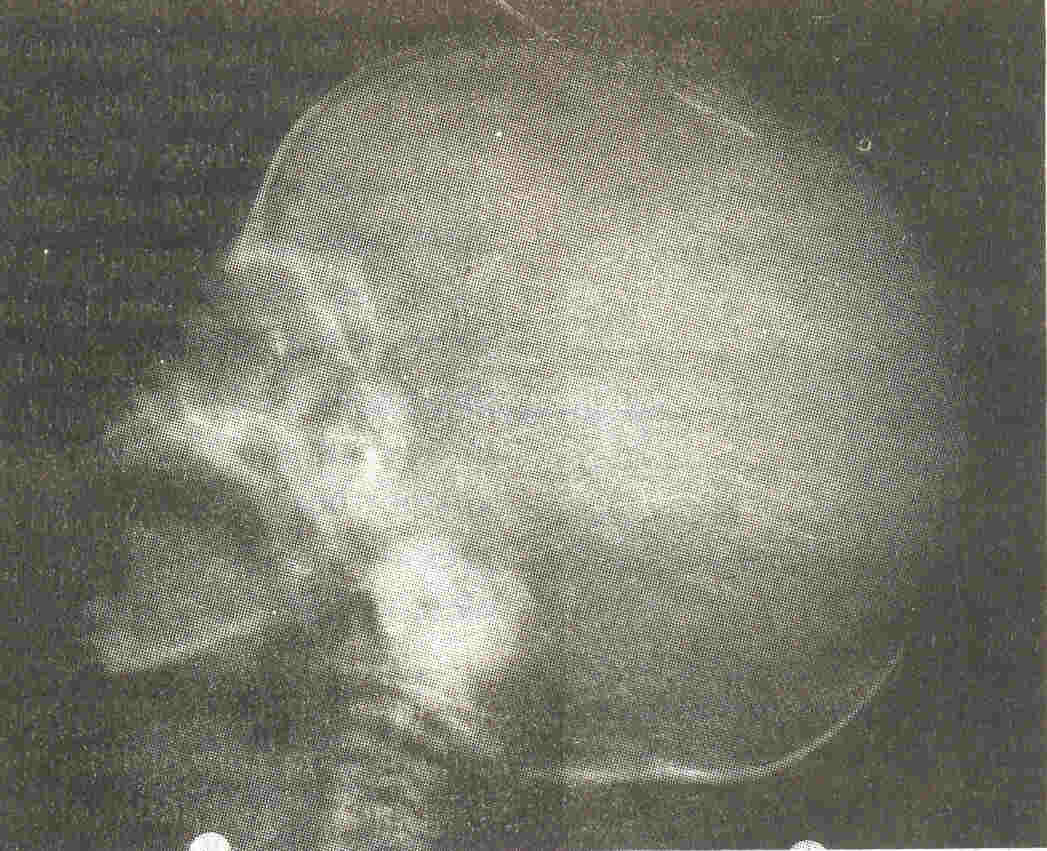 |
|
Fig. 1 X-ray skull showing hypocalvaria |
The patient was treated with fluid restriction, diuretics such as lasix and antibiotics namely cefotaxime sodium, ceftazidime, cloxacillin, ciprofloxicin and fluconazole. Peritoneal dialysis was started on day 7 of life within hours of admission. It was discontinued after 2 days in view of catheter blockage. Patient's biochemical markers showed improvement but he was still anuric - Peritoneal dialysis was thus restarted, however a tenkoff cathetel' was placed this time. On day
10 he passed a few drops of urine for the first time. and thereafter at
a rate of less than 0.5 ml/kg/h. On day 12 he went into respiratory
arrest and required mechanical ventilation for 5 days. His blood
pressure was always maintained. He was on continuous peritoneal dialysis
for 17 days until he died of generalized septicemia on day 27 of life. ACE levels were not possible. Karyotype was normal. Post mortem kidney biopsy was suggestive of renal tubular dysplasia.
Discussion
ACE inhibitors are inhibitors of kininase II and effect both the angiotensin/aldosterone and bradykinin/prostaglandin
systems. Excretion is basically by the kidneys. They are shown to cross the human placenta whereby they-are excreted in the amniotic fluid and re- cycled by swallowing in the fetus. In conditions
of low renal perfusion pressure, as exists in the fetus, the renin-angiotensin system plays a very important role and is essential in the maintenance of glomerular filtration and production of urine. The transplacental
passage of ACE inhibitors will result in a marked decrease in glomerular
filtration pressure with the attendant loss of urine production, manifest as oligohydramnios.
The most commonly reported adverse effects in ACE inhibitor exposed pregnancies are middle to third trimester onset of oligohydramnios, intra uterine growth retardation (IUGR), hypotension, anuria, hypocalvaria and pulmonary hypoplasia. The most probable and proved mechanism to explain the relationship of these features is an underlying fetal hypotension. Hypocalvaria, i.e., the calvarial bones although normal in shape and configuration, are remarkably small leaving the top third of the brain unprotected
by bone. This has been attributed to poor perfusion of the skull bones
rather than a defect in organogenesis(5). Although, persistence of patent ductus arteriosus may have an association, the high rate of this defect in premature infants makes the data inconclusive.
The word "Fetopathy" and not "Terato- genicity" is used, as till date there is no proof
of true teratogenic or first trimester effect of ACE inhibitors on the human embryo, and exposure during this time is not an indication for abortion. In contrast the risk of fetotoxicity has been proved without doubt with second and third trimester exposure, resulting in major morbidity and mortality(6).
In the cases reported till date, most of the mothers were detected to have second to third trimester onset oligohydramnios which progressed, requiring preterm delivery by LSCS. The newborns were usually IUGR with severe hypotension and anuria. This hypotension was found to be recalcitrant to both volume and pressor
therapy(7). Ultra-sound kidneys was normal, but biopsy showed renal
tubular dysplasia(6,7). Treatment consists of peritoneal dialysis. Enalapril and captropril are predicted to be somewhat dialysable,
because of their low protein binding capacity. Hemodialysis is preferable to peritoneal dialysis for faster drug clearance(4). Table I summarizes the cases of this condition reported till date and their outcome.
TABLE I-Summary of Earlier Cases.
Authors
|
No. of cases
in series |
IUGR
|
Oligohy-
dramnios |
Hypotension
and anuria |
Mortality
|
Plouin and
Tchobrousy(9)
|
7 case series
|
2/7
|
2/7
|
None noted
|
3/7
|
Kreft-Jais
et al,(8)
|
31 case series
|
9/31
|
Not recorded
|
Not recorded
|
3 Stillborn,
rest not recorded
|
Rose et al.(10)
|
4 case series
|
Weights
not
recorded
|
2/4
|
4/4
|
0/4, 2/4 have
chronic renal
insufficiency
|
|
Pryde et al. (7)
|
14 case
reports
(compiled)
|
9/14
|
10/14
|
9/14
|
8/14
|
|
Sedman et al.(4)
|
3 case series
|
3/3
|
3/3
|
3/3
|
2/3, 1/3 renal
transplant
|
Our patient had an evolving oligohydramnios leading to a preterm LSCS delivery, IUGR, neonatal anuria, and hypocalvaria. He had no hypotension. ACE,
levels unfortunately were not possible. Though the ultrasound kidneys were normal, postmortem kideney biopsy was suggestive of renal tubular dysplasia.
The possibility that the two spontaneous abortions the mother had prior
to this pregnancy could be due to the drug effect must also be entertained.
We thus conclude, that occasionally we come across a drug whose record in pregnancy is so frequently associated with an ad- verse outcome of so consistent a pattern that it becomes clear that it's use must be
restricted. We believe this to be the case with ACE inhibitors.
The best protocol to prevent ACE inhibitor fetopathy is to aggressively educate physicians and the public that this drug should not be used in second and third trimesters. of pregnancy. If inadvertent administration takes place it is estimated that 50% of infants will be affected and 25% will die(7). When
ACE inhibitor use in pregnancy outweights
the risk, careful fetal monitoring, for problems associated with oligohydramnios
is advised. The delivery should take place in an institute that can deal with prolonged renal failure and hypotension in the newborn. Whenever possible it is prudent to discontinue use of these drugs in women planning pregnancy and to avoid starting them during pregnancy.
Acknowledgement
We would like to thank Dr. N.C Joshi,
Dean, Bai Jerbai Wadi a Hospital for Children, for allowing us to publish this article.
|
1.
Materson 8J, Preston RA, Angiotensin converting enzyme inhibitors in hypertension, Arch Intern Med 1994; 154: 513-523.
2.
Guignard JP. Renal function in the newborn infant Pediatr Clin North Am 1982; 29: 777- 790.
3.
Piper JM, Ray WA, Rosa FW. Pregnancy outcome following exposure to angiotensin
converting enzyme inhibitors. Obstet Gynecol 1992; 80: 429-432.
4.
Sedman AB, Kershaw DB, Bunchman TE. Recognition and management of angiotensin
converting enzyme inhibitor fetopathy. Pediatr Nephrol 1995; 9: 382-385.
5.
Barr M, Cohen MM. ACE inhibitor fetopathy and hypocalvaria: The kidney-skull connection. Teratology 1991; 44: 485-495.
6. Barr M. Teratogen update: Angiotensin converting enzyme inhibitors. Teratology 1994;
50: 399-409.
7.
Pryde PG, Sedman AB, Nugent CE, Barr M. Angiotensin ,Converting enzyme inhibitor fetopathy. J Am Soc Nephrol1993; 3: 1575-
1582.
8.
Kreft-Jais C, Plouin C, Tchobroutsky C, Boutroy MJ. Angiotensin converting enzyme inhibitors during pregnancy: A survey of 22 patients given captopril and 9 given enalapril. Br J Obstet Gynecol 1988; 95: 420-422.
9.
Plouin PF, Tchobroutsky C. Inhibition de I' enzyme de conversion de I' angiotensine au cours de,la grossesse humaine, Quinze cas. Presse
Med 1985; 14: 2175-2178.
10.
Rosa FW, Bosco LA, Fossum-Graham C. Neonatal anuria with maternal angiotensin converting enzyme inhibition. Obstet Gynecol 1989; 74:.371-374.
|
