J.S. Whitehall, S.K. Patole and P. Campbell*
From the Department of Neonatology, Kirwan Hospital for Women, QLD 4817, Australia and the *Department of Neonatology, Royal Hospital for Women, Sydney NSW 2021, Australia.
Reprint requests: Dr. John Whitehall, Director, Department of Neonatology, Kirwan Hospital for Women, QLD 4817, Australia.
Manuscript received: September 23, 1997; Initial review completed: November
14, 1997; Revision accepted: September 16, 1998.
Abstract:
Objective: To evaluate safety and efficacy of recombinant human erythropoietin (r-HuEPO) in reducing the need for red cell transfusions in anemia
of prematurity: Methods: Forty-two preterm infants (gestational age ~2 weeks) were randomly assigned to a "treatment" group (r-HuEPO) 400 units/kg every alternate day x /0 dose5) or "no treatment" (control) group, All infants on enteral feeds received oral iron 3 mg/kg/day, graded up to 6 mg/kg/day. Results: Higher reticulocyte counts in week 2 and 3 and higher hemoglobin levels in week
4 were noted after treatment with r-HuEPO. Despite stimulated erythropoiesis, the frequency of transfusions could not be reduced with r-HuEPO therapy. Overall, phlebotomy losses, frequency and volume of red-cell
transfusions were significantly more in neonates with birthweight
≤1000
grams compared
with those with birthweight >1000 grams (p <0.05). Associated side effects of r-HuEPO such as neutropenia, sepsis, hypertension or increased risk of late death did not occur. Conclusions: r-HuEPO therapy was safe without any side effects. Inability of r-HuEPO therapy to minimize red- cell transfusions for anemia of prematurity may be explained by a relatively strict red-cell transfusion policy and the desired degree of treatment effect.
Key words: Anemia, Erythropoietin, Prematurity, Recombinant.
ANEMIA inpreterm infants is a common 'Occurrence, In the first
two weeks of life a major cause is the lass Of blood related to investigations or related
to delivery. Recent data shows that phlebotomy lasses correlate with the volume
of blood transfused, even in older and mare stable pre-term infants(1,2). Law levels
of hemoglobin are often noted between 2 and 6 weeks of age in infants barn after gestational age
of less than 35 weeks. A diminished erythropaietin response to anemia and available 'Oxygen plays a central role
in this hyporegenerative anemia. This is the classic normacytic, normochromic "anemia
of prematurity". A rapid rate of growth with resultant need for new red
cells arid a reduced life span of neanatal red cells also contribute towards it's develapment(3).
Inadequate erythropaietin production, pre-scnce of bane marrow erythroid hypaplasia and erythroid progenitors that respond
to erythropoietin in vitro form the physiological basis of use of recombinant human erythropaietin (r-HuEPO) in the management
of
anemia of prematuriy(4-10). The aim of this pilot study was to evaluate safety and efficacy of r-HuEPO in reducing the need for red cell transfusions in anemia of pre- maturity.
Subjects and Methods
Pre term infants with gestational age
≤32
weeks were considered eligible. for enrollment during the study period August.l992 to March 1993. Infants were excluded from the study in presence of the following conditions: (i) Major congenital anomaly; (ii) Primary hematological disease, (iii) Hypertension; (iv) Seizures; and (v) Failure to obtain consent. An informed parental consent approved by the Hospital ethics committee was obtained prior to enrollment of the infants at a postnatal age of 14 days. Enrolled infants were randomized to "treatment" with r-HuEPO
group or "no treatment" group using computer generated random numbers
contained in sealed, coded envelopes.
For ethical reasons the control group was assigned to "no treatment"
instead of subcutaneous injections of a placebo. Infants as-signed to
the "treatment" group received recombinant human erythropoietin (EPREXR, Janssen-Cilag) subcutaneously in the. dose of 400 units per kilogram every second day for a total of 10 doses. Infants assigned to the "no treatment" (control) group did not receive erythropoietin injections. The study infants were stratified into two groups. Group A included infants with birth weight
>1000 g and group B included infants with birth weight >1000.
Infants commenced on enteral nutrition also received daily oral iron at a dose of 3 mg/kg/day initially which was -then gradually increased to a maximum of 6 mg/kg/day. if tolerated to ensure optimal iron intake during r-HuEPO therapy(11).
In an effort to minimize iatrogenic blood loss, serum iron and ferritin levels were not done. Guidelines for red-cell transfusions for anemia of prematurity were based on the existing policy in the nursery, generally adopted by the neonatologists. They were as follows:
I. Transfuse Infants at Hb
≤8 g/dl (a) If reticulocyte
count is <4% -and (b) If receiving supplemental oxygen >30% or (c) If unexplained recurrent apneas-bradycardias are noted (> 1-2 per hour) or (d) If persistent tachycardia (heart rate > 170/min) or tachypnea (respiratory rate >60/min) is noted or (e) If there is failure 'to gain weight or successive weight loss
on weekly recordings for 3 consecutive weeks. In absence of a dear evidence in the literature justifying red cell transfusions at a hemoglobin of
≤8 g/dl in otherwise asymptomatic
neonates who are failing to thrive, it was decided that failure to gain weight or successive weight loss on weekly recordings for 3 consecutive weeks was a fair and substantial clinical indicator of the
need to transfuse(12).
II. Transfuse Infants at Hb
≤10 g/dl (a)
If receiving supplemental oxygen
≥30% and (b) needing intermittent mandatory ventilation (IMV) or continuous positive airway pressure (CPAP) by nasal prongs for recurrent (> 1-2 per hour) apneasebradycardias with saturations <90% on the pulse oximeter.
III. Transfuse Infants at Hb
≤12g/dl (a) If receiving mechanical ventilatory support
with Mean Airway Pressure (MAP)
≥10
and supplemental oxygen
≥30% during
the acute phase of illness after birth.
Data Collection
Phlebotomy losses for investigations and
the volume of red-cell transfusions were re- corded for each infant from delivery until time of discharge from the hospital during the study period. Full blood count and reticulocyte counts were monitored on a weekly basis. The incidence 'of sepsis (a positive blood culture with clinical deterioration) and hypertension (systolic blood pressure >90 mmHg) was also recorded during the study
period.
Statistics
Over a period of one year, prior to the commencement of our study, the
mean (±SD) number of, red cell
transfusions for anemia of prematurity in preterm infants
≤32 weeks gestation) was 4 (±2) per infant. A total sample size of 42 infants was estimated to be necessary to detect a 50% reduction in the number of transfusion per infant (primary outcome variable) with alpha, beta errors and power set at 0.05, 0,2 and 80%, respectively., The sample size allowed for a 10"15% drop out rate due to withdrawal of consent, loss, of data, etc. Post-randomization reticulocyte count and hemoglobin were the secondary outcome variables of interest.
The Mann-Whitney test was used to anacIyze differences between the two study
groups.
Results
A total of 42 infants including 16 males and 26 females were randomized. Of the twenty infants in Group A, 10 received r- HuEPO and 10 did not. Of the twenty-two,
infants in Group B, 12 received r-HuEPO and 10 did not. Tile mean (±SD) birth weight of infants in Group A who received r-HuEPO was 779±126 vs 807±153 in those who did not receive treatment with r-HuEPO.
The mean (±SD) birth weight of
infants in Group B who received r-HuEPO was 1233±77 g vs 1189±178
g in those who did not receive it. The mean (±SD) gestational age of infants in Groups A and B was 26 (±2) and 30 (±2) weeks, respectively. Within the Groups A or B, there was no significant difference in the ,distribution of gestational ages of "study" (r-HuEPO therapy) or "control" (no r-HuEPO therapy) infants. Respiratory distress syndrome (Group A: n=18,
Group B:=16) and probable transient
tachypnea of the newborn (Group A: n
=
2, Group B: n =, 6) were the two ,illness ob- served in the infants prior to enrollment. Nosocomial
sepsis and chronic lung disease were the common illnesses noted during
the nursery stay in both the study and control group of infants after
enrolment in the study.
Red cell Transfusions
There were no differences in the number of transfusions given to babies, or the total amount transfused after randomization, in the treated or untreated group of babies, regard- less of their weight (Table I). Blood loss by phlebotomy in the treated and untreated babies was not significantly different. Irrespective of the treatment status there was a significant difference in the number of transfusions, the ratio of amount transfused to either the blood loss or the birthweight and the total amount transfused to babies
≤1000g 'compared with those exceeding that weight (p <0.05).
Reticulocyte Count
The reticulocyte count was expressed as a percentage of red cells and records were averaged
over each week pre and post randomization.
|
TABLE I-Post-Randomization Transfusion
and Blood Loss Data (Mean±SD)
|
|
|
Number of
transfusions
|
Amount
transfused
(ml)
|
Amount of
blood loss (ml)
|
Amount
transfused
(ml)/Blood
Loss (ml) |
Amount
transfused
(ml)/Birth
weight (kg) |
|
By Weight |
|
|
|
|
|
|
≤1000 g (n
=
20)
|
3.3±2.0
|
66.4±39.9 |
35.6±19.4 |
1.92±1.12 |
87.7±55.5 |
|
>1000 g (n
=
22)
|
0.7±1.2
|
19.8±32.0 |
] 2.5±10.5
|
1.04 ± 1.65.
|
17.4±28.9 |
|
|
(p <0.0001) |
(p
=
0.0002)
|
(p <0.000]) |
(p
=
0.0) 7)
|
(p<0.0001)
|
|
By Treatment |
|
|
|
|
|
|
r-HuEpo (n
=
22)
|
1.7±2.0
|
35.3±37.5 |
25.0±20.7 |
0.99±0.99 |
45.6±56.0 |
|
no r-HuEpo (n
=
20)
|
2.2±2.1
|
49.4±47.5 |
21.8±17.7 |
1.98 :!: 1.75 |
56.7±56.5 |
|
|
NS |
Ns
|
NS
|
(p
=
0.06)
|
NS |
|
≤1000 g Group |
3.2±1.9 |
64.0±29.3 |
41.0±18.5 |
1.63±0.71 |
89.1±54.3 |
|
r-HuEpo (n
=
10)
|
3.3±2.2
|
68.9±49.9 |
30.1±19.6 |
2.21±1.39 |
86.4±59.S. |
|
no r -HuEpo (n
=
10)
|
NS
|
NS |
NS |
NS |
NS |
|
>1000 g Group |
0.4±0.9 |
11.4±24.9 |
11.6±10.4 |
0.45± 0.9 |
9.4±20.5 |
|
r-HuEpo (n
=
12)
|
1.1
±1.4
|
29.9±37.7
|
13.5±11.0
|
1.7±2.1 |
27.1±35.3 |
|
no r-Hu Epo (n
=
10)
|
NS
|
NS |
NS |
NS |
NS |
P
=
Values were calculated by Mann Whitney test.
NS
=
Not significant. |
Overall, the mean (±SD) reticulocyte count for the
7 day period prior to randomization was not significantly different when all infants treated with r-HuEPO were compared with all those not treated with
r-HuEPO. (1.9±1.2% vs 1.8±1.3%, P >0.05) (Table II). The prerandomization reticulocyte counts were also not significantly different when the data was analyzed after stratifica- tion by birth weight and randomization status (Fig. 1). The values were: Group A: [r-
HuEPO vs no r-HuEPO
=
1.6 (±o.7)% vs 1.2
(±0.9%); P >0.05] and Group B: [r-HuEPO vs no r-HuEPO
=
2.1 (±1.1)% vs 2.5 (:to.7)%; P >0.05]. Elevations in reticulocyte counts were observed in the babies treated with r-HuEPO compared with those not treated (Table II) at week 1 (2.5 vs 1.1; p
=
0.009) and week 2 (8.2 vs 2.4 P
=
0.0001) of treatment and at week 3 post-randomization
.
Le., a week after cessation of treatment (5.9 vs 2.6; p =0.003) (Table II). Analysis of the data after stratification by birth weight
(Group A:
≤1000 Group B: >1000) showed a similar trend in significant rise in reticulocyte count at weeks 1, 2 and 3 post randomization in both Groups A and B infants treated with r-HuEPO. The maximum rise occurred at week 2 after randomization in these' infants (Fig. 1).
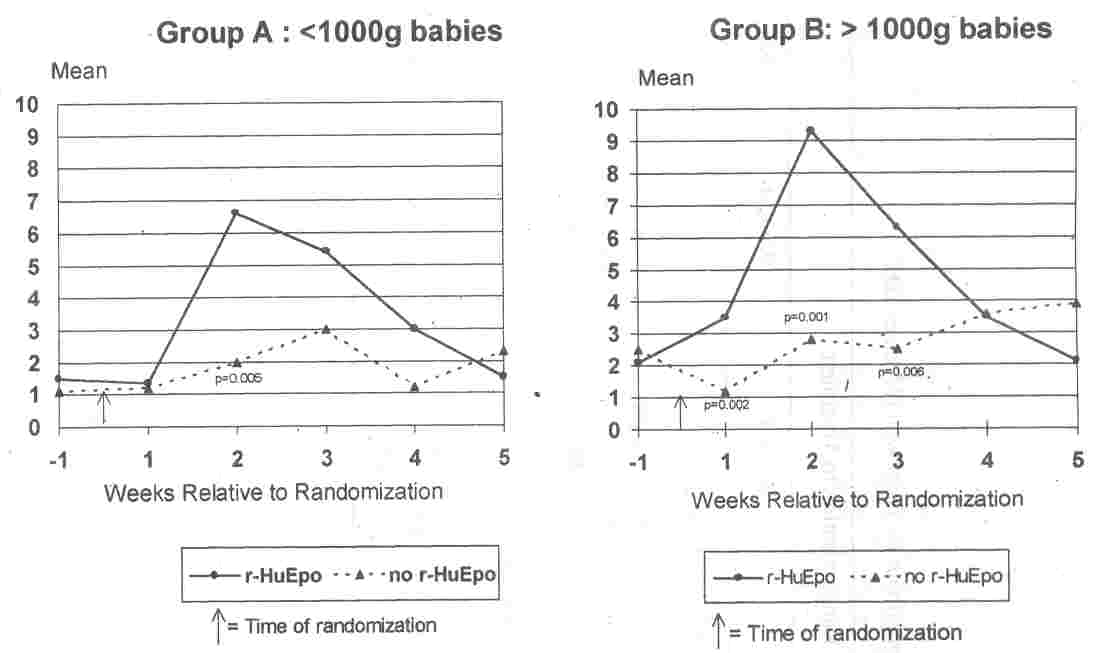 |
|
Fig. 1. Average weekly reticulocyte count computed as"% red blood cells
for the week preceding randomization and 5 weeks post-randomization. The time points
at which there were statistically significant differences between treatment and non-treatment are noted with the
relevant p values. |
Post Randomization Phlebotomy Losses and' Volume of Red
Cell
Transfusions
The total amount of blood loss post ran- domization in Group A (≤
1000 g) infants was significantly more compared to the heavier infants in Group B (Table
I) (35..6±19.4 vs 12.5
±10.5; P <0.05). Similarly frequency and volume of red cell transfusions were also more in Group A infants (3.3±2 vs 0.7±1.2 transfusions; p <0.05 and
66.4±39.9 vs 19.8±3.2 ml transfused, p <0.05).
TABLE II
Average Weekly Reticulocytes and Hemoglobin by Treatment (Mean±SD).
| |
Week Relative to
Randomisation to Treatment |
| |
Week -1 |
Week 1 |
Week 2 |
Week 3 |
Week 4 |
Week 5 |
| |
Reticulocytes (Average Weekly
% RBC) |
| R-HuEpo (n=22) |
1.9±1.2(17) |
2.5±1.7(18) |
8.2±4.3(19) |
5.9±3.4(20) |
3.3±1.8(16) |
1.8±1.3(17) |
| no R-HuEpo
(n=20) |
1.8=1.3(15) |
1.1±0.6(13) |
2.4±1.9(17) |
2.6±2.6(16) |
2.5±1.9(20) |
3.1±3.2(14) |
| p (t-test) |
NS |
0.009 |
0.0001 |
0.003 |
NS |
NS |
| |
Hemoglobin (Average Weekly
g/dl) |
| R-HuEpo (n=22) |
13.5±2.2(22) |
13.2±2.0(22) |
13.0±2.0(21) |
13.0±1.6(22) |
12.7±1.7(21) |
11.7±1.1(20) |
| no R-HuEpo
(n=20) |
14.2±3.0(19) |
13.0±2.1(19) |
12.2±1.8(19) |
12.3±1.9(18) |
11.3±1.5(17) |
11.5±1.9(17) |
| p (t-test) |
NS |
NS |
NS |
NS |
0.01 |
NS |
Figures in parantheses indicate percentages.
NS-Not significant.
Hemoglobin
Overall, the mean (±SD) hemoglobin levels over a weeks period prior to randomization were not significantly different when all infants treated with r-HuEPO were compared with all those not treated with r-HuEPO. [13.5(±2.2) g/dl vs 14.2 (±3) g/dl, p >0.05] (Table II). The pre randomization hemoglobin
levels were also not significantly different when the data was analyzed after stratification by birth weight and randomization status. The values were Group A: [r-HuEPO vs no r-HuEPO
=
13.2 (±2.1)
g/dl vs 13.8 (±
1.8) g/dl, p >0.05] and Group B: [r-HuEPO vs no
r-HuEPO
=
13.3 (±1.8) g/dl vs 14 (±1.7)
g/dl; p >0.05].
In the fourth week after randomization
there was a significant elevation in hemoglobin levels when all babies treated with r-HuEPO were. compared with all those not treated with r-HuEPO [12.7 (±1.7) g/dl vs 11.3 (±1.5) g/dl; P <0.05]. (Table II). Data analysis after stratification by birth weight (Group A:
≤1000 g, Group B: >1000 g) showed a similar significant rise in hemoglo- bin levels in Group B babies treated with r-HuEPO in the fourth week post-randomization [13.1 (±1.5) g/dl vs 10.7 (±1.4) g/dl; p
=
0.001]. The levels were not different at any other time in the two study groups regardless of the randomization status.
White Cells and Platelets
The average weekly white cell counts and platelets were not significantly different in
the r-HuEPO treated babies compared with the babies not treated, during the week
prior to randomization or for the 5 weeks following randomization.
Iron Therapy
There was no significant difference in the duration of oral iron therapy in study groups A or. B when r-HuEPQ treated infants were compared with untreated ones. However, there was a statistically significant difference between the amount of iron given to babies in Group A compared to those in Group B. Group A infants received iron 3.4±2.2 mg/ week vs 5.1±2.4
mg/week, in Group B infants (p
=
0.02).
Nutritional Details
'Out of the 16 survivors in a total of 20 Group A infants there Was no
significant difference in time taken to commence and reach full enteral feeds (150 ml/kg/day of breast milk or 20 calories/30 ml formula) [Study: 10.34
±4.5 vs Control: 11.38±2.8 days for commencing feeds, Study: 12.38±2.5 vs Control: 13.4±3.1 days for reaching full feeds; p >0.05]. Of the 4 deaths in Group A (ore
in treated and three in untreated) none reached full feeds. Similarly, in Group
B there was no significant difference in the time taken to commence and reach
full enteral feeds (Study: 6.42±2.45 vs Control:
8.12±1.2 days for commencing feeds; Study 9.38 ±2.56 vs Control: 11.52±7.57 days to reach full feeds; p >0.05).
Adverse Events
No adverse events related to treatment were reported. Specifically, there was no difference in the incidence of sepsis, nuetropenia or hypertension. Four deaths occurred in Group A. One baby died from respiratory failure secondary to chronic lung disease in the treated group. Three babies
died in the untreated group, one from over- whelming sepsis, one from respiratory failure secondary to chronic lung disease and an- other from presumed milk aspiration.
Discussion
Treatment with r-HuEPO was associated with a stimulatory effect on erythropoiesis. The increase in reticulocyte production
occurred
one week after commencing
r-HuEPO and persisted till one week after cessation of therapy. The stimulatory effect of r-HuEPO
was best observed when the total volume of red-cell transfusion after randomization was compared with the phlebotomy losses during the same period. It was found that less volume of blood was needed to reach a desired level of hemoglobin in those treated with r-HuEPO, implying that erythropoiesis
was stimulated to such a degree in these infants that a smaller volume of blood needed to be transfused to obtain the' desired hemoglobin levels.
The rise in hemoglobin occurred mainly during the fourth week of r-HuEPO therapy. Despite the stimulatory effect on. erythropoiesis, there were no significant differences in the frequency or total volume of red-cell transfusions given to study infants in the post randomization period irrespective of their randomization status. Large volumes of
blood removed for monitoring these 'at risk' infants may explain why many r-uEPO treated infants received at least one or more blood transfusions. Phlebotomy losses were significantly different in Group A compared with Group B infants (35.6±19.4 vs 12.5±10.5 ml; p =0.0001). There was also a significant difference in the frequency and total volume of red-cell transfusions given to babies in Group A compared with those in Group B (3.3±2 vs 0.7±1.2 transfusions;
p
=
0.0001 and 66.4±39.9 ml of red cells
transfused vs 19.8±32 ml; p
=
0.0001). Frequent phlebotomies and proportionately larger blood loss due to a
low overall blood volume in these smallest and often the sickest infants may explain these differences. A blunted erythropoietic
response due to extreme prematurity, a fairly strict and objective transfusion policy and poor nutritional intake (especially iron and proteins) may ex- plain failure of r-HuEPO in reducing the frequency
or volume of red-cell transfusion in these infants after randomization. Serum
iron levels were not measured in this study, in order to minimize phlebotomy
losses but are
likely to have been less than optimal in these smallest infants. Adequate levels of iron and an adequate intake of proteins is known to be essential for maximal response to r-HuEPO(l3,14). The bigger infants in Group B (Birth weight>1000) responded better to r-HuEPO, their reticulocyte counts, hemoglobin levels were higher, and they needed to receive less blood per unit lost to maintain a desired level of hemoglobin. A comparatively
healthier condition resulting in diminished phlebotomy losses, fewer derangements in oxygen delivery and greater tolerance for low hemoglobin levels may be the reasons for better response to r-HuEPO
in these infants. Indeed the time taken to commence and reach full enteral feeds assuring adequate protein-caloric intake was less in. Group B infants resulting in effective erythropoiesis by r-HuEPO.
Results of three large randomized controlled trials of r-HuEPO in preterm infants have been published recently(1,15,16). Maier et. at. reported higher reticulocyte counts and a reduction in the volume of red cell transfusions with r-HuEPO treatment in a study of 241 preterm infants(15). In their study, r-HuEPO was used at a dose of 250 units/kg subcutaneously, three time a week from day3 to day 42 for a total of 17 doses. Liberal transfusion criteria, lack of a placebo group and a small number of infants in the study population at risk for receiving multiple transfusions were the inherent problems in this study. Our data differs from the
results of Maier et at. in that we did not find an in- creased incidence of sepsis in the r-HuEPO treated infants though .our sample size was smaller( 15). Although, phlebotomy losses were not reported by Maier et al. the volume of blood sampled for laboratory tests was
substantial;0.74 and 0.83 ml/kg/day in control and EPO-treated infants(l5) Meyer et al. conducted a double-blind, placebo-controlled study of r-HuEPO in the treatment of anemia of prematurity in 80 relatively large infants(16). Subcutaneous r-HuEPO
at a dose of.600 units/kg per week was used for up to 6 weeks. A significant
reduction in the number and volume of red-cell transfusions, higher reticulocyte counts and hematocrits were observed in r-HuEPO treated group. None of the study infants required assisted ventilation and the mean phlebotomy loss was less
than 8 ml per patient during the study period. Maturity of the enrolled infants with resultant low phlebotomy losses may explain the overall low rate of transfusion. No significant increase in incidence of infections or neutropenia was reported by Meyer et at., similar to our results(16). Shannon et at. have recently reported the results of a double-blind, placebo-controlled. multi centre trial of r-HuEPO in 157 preterm infants(1). The dose of r-HuEPO was 100 units/kg/day, 5 days per week for 6 weeks. Stimulation of erythropoiesis, moderation of the course of anemia and reduction in red-cell transfusions was reported. No adverse effects like neutropenia,
infections, hypertension and increased risk of late death related to rHuEPO therapy were reported by them. Merchant et al in a prospective uncontrolled study inIndia, treated 10 infants with r-HuEPO(17). The criteria for using r-HuEPO were gestational age
≤32 weeks, hemolgobin level
≤7.5g/dl and/or hematocrit
≤24% and a reticulocyte count
≤3% r-HuEPO was used at
a dose of 200 units/kg/dose thrice a week for three weeks for a total of 10
doses. All infants also received oral iron in the dose of 6 mg/kg/day. A significant rise in hemoglobin and retitulocyte count was noticed after therapy with r-HuEPO. Blood transfusion was required in four infants who did not show a significant rise in either hemoglobin or reticulocyte count. No adverse effects or significant effects on white cell or platelet count were noted.
No side effects related to iron therapy were observed in our study. Our maximum dose of iron at 6 mg/kg/day is different as compared to that reported by Carnielli et al. who administered 20 mg of iron/kg/week intravenously without complications( 18). Bechteen et al. also used intravenous iron apparently without any complications(19). The difference in the total amount of iron received and the duration of iron therapy in the two groups could be explained by the frequent episodes of feed in tolerance in smaller (Group A) babies needing stoppage of enteral intake.
It would appear timely to reassess traditional fears of iron therapy. The. problem with iron therapy would now appear to reside more in the decision of how much should be given and by which route, rather than with side effects. Its side effects may be less worrisome compared to blood transfusion related risks. Inclusion of iron in parenteral nutritional fluids in doses guided by serum levels may be appropriate in infants intolerant of oral feeds.
The neutrophil counts, leukocyte counts
and platelet counts were not significantly different in our study group infants
before or after randomization, irrespective of their randomization status.
Treatment with r-HuEPO has been associated with neutropenia and granulopoiesis in some trials but not in others(20-21). Our results indicate normal neutrophil counts during periods of vigorous erythropoiesis in preterm infants. Cost
analysis comparing erythropoietin and red-cell transfusions in the treatment of anemia of prematurity have been reported(25,26). Fain et at.
reported that the base-case cost in 1993 U.S. dollars for treating anemia in
hospitalized premature infants with erythropoietin and transfusions was
$1,326(25). This was nearly twice the cost of conventional treatment with transfusions alone ($721). Shireman et al. using assumptions based on state of clinical research in 1994, reported that routine use of r-HuEPO with supplemental RBC transfusions would not generate any cost savings as an alternative to RBC transfusions alone(26). Their base case analysis yielded a net loss of $299.48 per infant. A 54% reduction in the direct product costs of r-HuEPO (unlikely at present) would yield a break-even point. The implications of these reports are important for developing countries where the issue of cost overrides many priorities. The current cost of treating a 1000 g infant with 10 doses of r-HuEPO in India while following our regime will be approximately Rs.10200 if the "single-use only" vials ("EPREX Ethnor) containing 2000 units/ml of r-HuEPO are used. The cost of the therapy could be reduced significantly by separating the contents of the vial into multiple doses under the aseptic "laminar flow" technique but is not recommended by the drug manufacturer. Considering that the relatively large or stable preterm infants who respond best to r-HuEPO and iron need relatively few red cell transfusions their
need for
r-HuEPO to avoid/minimize transfusions is minimal. Unfortunately, extremely low birth weight infants who are sick and have the greatest need for red cell transfusions shortly after birth, have not consistently responded to r-HuEPO and iron. Thus, those wishing to prescribe r-HuEPO are still in a dilemma. Given that medical decisions ate often influenced by cost in the developing countries a strict, objective policy for transfusions for anemia of prematurity, minimizing phlebotomy losses and limiting multiple donor exposure will. lead to reduction in the frequency of such transfusions and the need for r-HuEPO therapy(27).
We conclude that r-HuEPO treatment (400 units/kg every second day x 10 doses)
beginning at the postnatal age of 14 days was associated with stimulated erythropoiesis and higher levels of hemoglobin. A significant reduction in the frequency of red-cell transfusions following r-HuEPO treatment was not observed. A relatively strict red-cell transfusion policy, a desired 50% reduction in frequency of red cell transfusions and lack of blinding may explain our findings.
|
1.
Shannon. KM, Keith JF, Mentzer WC, Ehrenkranz RA. Brown MS, Widness JA, et al. Recombinant" human erythropoietin stimulates erythropoiesis and reduces erythrocyte transfusions in very-low birth weight preterm infants. Pediatrics 1995; 95: 1-8.
2. Shannon KM. Mentzer WC, Abels RI. Freeman P, Newton N, Thompson D, et al.
Recombinant human erythropoietin in the. anemia of prematurity: Results of a placebo~con-
trolled pilot study. J Pediatr 1991; 18:
949-
955.
3.
Pearson H. Life span of the fetal red blood cell. J Pediatr 1967; 70: 166.
4.
Shannon KM. Anemia of prematurity: Progress and prospects. Am J Pediatr Hematol Oncol 1990; 12: 16-22.
5.
Stockman JA Ill, Garcia JF, Oski FA. The anemia of prematurity: Factors governing the erythropoietin response. N Engl J Med. 1977; 296: 647-650.
6.
Brown Phibbs RH, Garcia JF. Dallman PR. Decreased response of plasma immunoreactive erythropoietin to "available oxygen" in anemia of prematurity. J Pediatr
1984;. 105: 793-798.
7.
Stockman JA III, Graeber JE. Clark DA. McClellan K. Garcia JF, Cavey REN. Anemia of prematurity: Determinants of erythropoietin. response. J Pediatr 1984; 105: 786-792.
8.
Gairdner D, Marks J, Roscoe JD. Blood formation in infancy. IV. The early anemia of prematurity. Arch Dis Child 1955; 30: 203- 211.
9.
Shannon KM, Naylor GS, Torkildson JC, Clemons GK, Schaffner Y, Goldman SL. et
al. Circulating erythroid progenitors in the anemia of prematurity. N Engl J Med 1987; 317: 728-733.
10.
Rhondeau . SM, Christensen RD, Ross MP, Rothstein G, Simmons MA. Responsiveness to recombinant erythropoietin of marrow erythroid progenitors in infant with anemia of prematurity. J Pediat(l988; 112: 935-940.
11.
Shannon K. Recombinant human erythropoietin in neonatal anemia. Clin Perinatol 1995; 22: 627-640.
12.
Strauss RG. Red blood cell transfusion practices in the newborn. Clin Perinatol 1995; 640-655.
13.
Eschbach JW, Egrie JC, Downing MR. Brown JK, Adamson JW. Correction of the anemia of .end-stage
renal disease with recombinant human erythropoietin.
N
Engl J Med 1987; 316: 73-78.
14. Ronnholm KAR, Siimes MA. Hemoglobin concentration depends on protein intake in small preterm infants fed human milk. Arch Dis Child 1985; 60: 99-104.
15.
Maier RF, Oblanden M, Scigalla P, Linderkamp O, Due G, Hieronimi G, et al. The effect of epoietin beta (recombinant human erythropoietin) on the need for transfusion in very-Iow-birth-weight infants. N Engl J Med 1994; 330: 1173-1178.
16.
Meyer MP, Meyer JH, Commerford A, Mary Hann F, Sive AA, Maller G. Recombinant human erythropoietin in treatment of the anemia of prematurity: Results of a doble-blind, placebo-controlled study. Pediatrics 1994; 93: 918-923.
17.
Merchant RH, Sonigara S, Sanghvi KP. Erythropoietin therapy for anemia of prematurity. Indian Pediatr 1996; 33: 323-326.
18. Carnielli V, Montini G, Riol RD, Dall' Amico R, Cantanitti F. Effect of high doses of human recol11binanterythropoietin on the need
for blood transfusions in preterm infants. J Pediatr 1992; 121: 98-102.
19.
Bechensteen AG, Haga p, Halvorsen S, Whitlaw A, Liestol K, Lindemann R, et at al.
Erythropoietin, protein, and iron supplementation and the prevention of anemia of prematurity. Arch Dis Child 1993; 69: 19-23.
20.
Halperin DS, Wacker P, Lacount G, Felix M, Babel JF, Aapro M, et al. Effects of recombinant human erythropoietin in infants with the anemia of prematurity. J Pediatr 1990; 116: 779-786.
21.
Beck D, Masserey. E, Meyer M, Calame A. Traitement intraveineux de l'anemie du premature par erythropoietine humanine recombinee [Abstract]. Schweiz Med Wochenschr 1990; 120 (suppl 34):
11A.
22.
Ohls RK, Christensen RD. A randomized trial of erythropoietin administration versus erythrocyte transfusion in patients with the anemia of prematurity [Abstract]. Clin Res 1991; 39: 12A.
23.
Shannon KM, Mentzer WC, Abels RI, Freeman P, Newton N, Thompson D, et al.
Recombinant human erythropoietin in theanemia of prematurity: Results of a placebo-controlled pilot study. J Pediatr 1991; 118: 949- 955.
24.
ObIaden M, Maier R, Segerer H, Grauel EL, Holland BM, Stewart G, et al. Efficacy and safety of recombinant human erythropoietin to prevent the anemia of prematurity. Contrib Nephrol 1991; 88: 314-326.
25.
Fain J, Hilsenrath PE, Widness
JA, Strauss RG, Mutnick AH. A cost analysis comparing erythropoietin and red cell. transfusions in the treatment of anemia of prematurity. Transfusion. 1995; 35: 936-943.
26. Shireman TI, Hilsenrath PE, Strauss RG, Widness JA, Mutnick AH. Recombinant human erythropoietin vs transfusions in the treatment of anemia of prematurity: A cost- benefit analysis. Arch Pediatr Adolesc Med. 1994; 148: 582-588.
27.
Strauss RG. Recombinant erythropoietin for the anemia of prematurity: StilI a promise, not a panancea. J Pediatr 1997; 131: 653- 655.
|
