|
|
|
Indian Pediatr 2021;58:162-168 |
 |
Comparative Efficacy
and Safety of Non-Steroidal Anti-Inflammatory Drugs in Patients
With Juvenile Idiopathic Arthritis: A Systematic
Review and Network Meta-analysis
|
|
Chun-lian Shi, Yu Zhang, Zhi-yong Zhang, Juan Zhou and Xue-mei Tang
From Department of Rheumatology and Immunology, Ministry of Education
Key Laboratory of Child Development and Disorders, National Clinical
Research Center for Child Health and Disorders, China International
Science and Technology Cooperation Base of Child Development and
Critical Disorders, Chongqing Key Laboratory of Child Infection and
Immunity, Children’s Hospital of Chongqing Medical University,
Chongqing, PR China
Correspondence to: Dr. Xue-mei Tang, Department of Rheumatology and
Immunology, Children’s Hospital of Chongqing Medical University, Yuzhong
District, Chongqing 400 014, China.
Email:
[email protected]
|
Objective: We conducted a systematic
review and network meta-analysis to compare the efficacy and safety of
nine non-steroidal anti-inflammatory drugs (NSAIDs) in treating patients
with juvenile idiopathic arthritis (JIA). Methods:
Randomized controlled trials (RCTs) of NSAIDs for the treatment in
children with JIA were searched systematically by using MEDLINE, EMBASE,
and the Cochrane Library for available literature up to January 1, 2019.
Bayesian network meta-analysis was used to combine direct and indirect
evidence on treatment effectiveness and safety. Results:
Eight eligible RCTs involving 1112 patients with JIA were identified,
addressing 9 interventions. The ranking probability plot based on the
surface under the cumulative ranking curve (SUCRA) indicated that
celecoxib (6 mg/kg twice-a-day) had the highest probability of being
most effective (SUCRA = 76.4%) among four NSAIDs (celecoxib, rofecoxib,
meloxicam, and naproxen). Also, rofecoxib (0.3 mg/kg once-a-day) and
piroxicam demonstrated a higher probability of safety in treating
children with JIA (SUCRA = 33.0% and 35.5%, respectively), compared with
other interventions. Conclusions: The quality of available
evidence limits the formation of powerful conclusions regarding the
comparative efficacy or safety of NSAIDs used to treat JIA.
Keywords: Drugs, Juvenile chronic arthritis, Management, Pain,
Rheumatoid arthritis, Side-effects.
|
|
J
uvenile idiopathic arthritis (JIA) is the
most common chronic rheumatic disease in childhood and one of
the leading causes of pediatric acquired disability. It
encompasses a heterogeneous group of disorders characterized by
chronic arthritis, of unknown etiology, lasting for 6 weeks or
more, with disease onset before 16 years of age having excluded
arthritis caused by other diseases [1]. Treatment is aimed to
achieve disease remission, prevent or halt joint damage, and
foster normal growth and development. Currently, early diagnosis
and treatment of JIA with conventional and biologic
disease-modifying anti-rheumatic drugs (DMARDs) have vastly
improved outcomes for children with these diseases.
Nonsteroidal anti-inflammatory drugs (NSAIDs)
are recommended as an adjunct therapy for symptomatic
management, particularly during initiation or escalation of
therapy with DMARDs or biologic agents [2]. NSAIDs exert their
analgesic and anti-inflammatory effects by blocking
prostaglandin formation via inhibition of cyclo-oxygenase
(COX) isoenzymes, a rate-limiting enzyme in the prostaglandin
biosynthetic pathway. Both non-selective (which suppress both
COX-1 and COX-2 enzymes) and selective (suppress COX-2 only)
NSAIDs have been used in JIA [3].
Previous comparative studies of NSAIDs were
mostly performed to evaluate the efficacy and safety of two
NSAIDs or one NSAID versus placebo [4,5]. However, the preferred
NSAID in the treatment with JIA still remains unclear. To
comprehensively compare and rank different NSAIDs in the
treatment of children and adolescents with JIA, we conducted a
systematic review and network meta-analysis [6,7].
METHODS
This systematic review with meta-analysis was
conducted and reported according to the Preferred reporting
items for systematic reviews and meta-analysis (PRISMA)
guidelines [8].
Eligibility criteria and search strategy:
Randomized controlled trials (RCTs) were included if they met
the following criteria: (i) the study compared any NSAID
with placebo or another NSAID in the treatment of JIA; (ii)
the study provided endpoints for the efficacy or adverse events;
and (iii) the study included patients diagnosed with JIA.
The details of eligibility criteria are provided in Web
Table 1. For this network meta-analysis, MEDLINE (via
PubMed), EMBASE, and the Cochrane Library were searched for RCTs
published from January 1, 1965, to January 1, 2019, comparing
the efficacy and (or) safety of NSAIDs in the treatment of JIA.
The following search terms were used: "#1 "Juvenile Idiopathic
Arthritis" OR "Arthritis" OR "Still Disease" OR "Rheumatoid" AND
#2 "NSAIDs" OR "Agents" OR "Non-Steroidal Anti-Inflam-matory
Drugs" OR "Analgesics" OR "indomethacin" OR "naproxen" OR "Naprosyn"
OR "aspirin" OR "acetylsalicylic acid" OR "celecoxib" OR
"Celebrex" OR "rofecoxib" OR "piroxicam" OR "ibuprofen" OR "meloxicam"
OR "tolmetin" OR "diclofenac" OR "Voltaren" OR "voltarol" AND #3
"randomized controlled trial" OR "controlled clinical trial" OR
"placebo" OR "drug therapy" OR "groups". In order to ensure the
authenticity of data, we also searched the list of references
included in the articles.
Data extraction: The following
information was extracted from each study: i) study
characteristics (author, year, study design); ii) patient
characteristics (sample size, race, age, sex, duration of JIA,
subtype); iii) interventions: any NSAID, dosage,
concomitant therapy, follow-up time when outcomes were
evaluated; and, iv) outcomes (efficacy, and adverse
events). Data were extracted from original studies by two
independent investigators, and any discrepancy between the
investigators was resolved by after discussions along with a
third investigator.
The primary outcomes were efficacy (the
number of patients who had improvement in symptoms of arthritis)
and safety (the number of patients who experienced adverse
events).
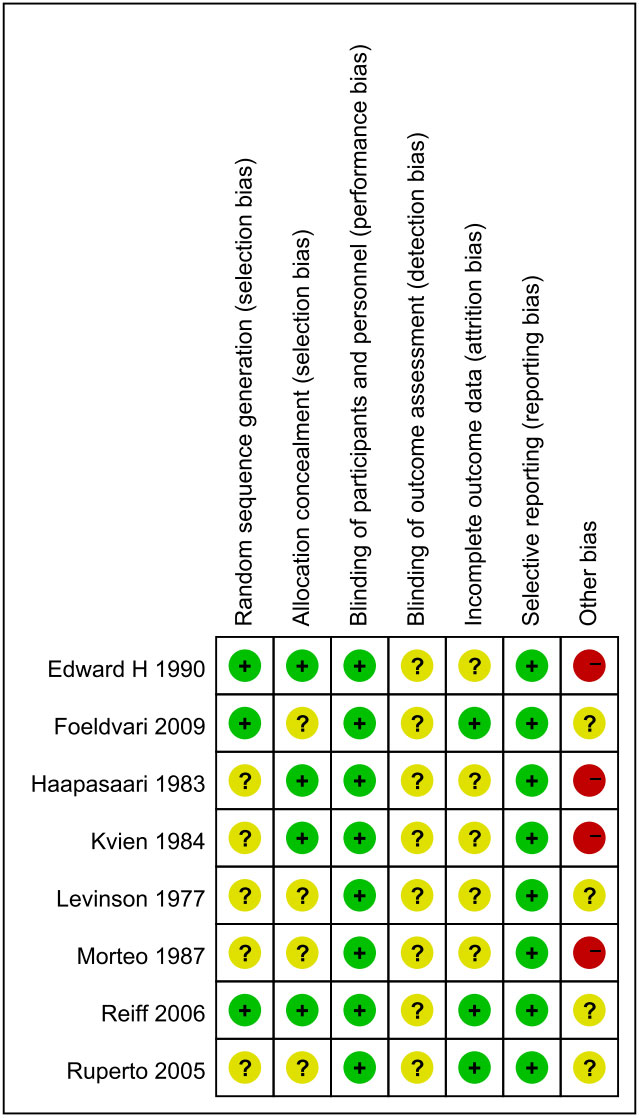
Quality assessment: The risk of bias was
assessed using Cochrane risk of bias tool [9], evaluating for
random sequence generation, allocation concealment, blinding of
participants and personnel, blinding of outcome assess-ment,
incomplete outcome data, selective reporting, and other bias
like sample size, [10] (labeling them as low risk of bias with
200 participants or more per treatment group, unclear risk with
50 to 199 participants per treatment group, or high risk with
fewer than 50 participants per treatment group), multiple
publications, financial declara-tions, and participants with
conflicts of interest.
Statistical analyses: First, we
divided the three-arm or more-than-three-arm tests into
combinations of any two arms, and performed the evidence network
diagram for the comparison of each treatment. We assessed the
inconsis-tency or extent of disagreement between direct and
indirect evidence. For closed loops, we tested the transitivity
assumption by examining loop-specific consistency between direct
and indirect effects using network side splits and global
consistency by comparing a model assuming consistency with an
inconsistent model. When the global Wald test indicated no
significant differences between the consistency and
inconsistency models [11] and no significant differences in
estimates based on side splits, we presented consistency model
estimates. The Markov chain Monte Carlo (MCMC) method was used
to obtain pooled effect sizes [6]. We calculated the risk ratio
(RR) with 95% credible interval (CI; or Bayesian confidence
interval). The efficacy and safety of NSAIDs in different arms
were ordered according to the probability of being ranked as the
best performing regimen. We did a network meta-analysis within a
Bayesian framework with WinBUGS (version 1.4.3) and further
analysis with Stata (version 15.1). Information on relative
effects and safety was converted to a probability that a
treatment is the best, second best, etc., or to the ranking of
each treatment, called the surface under the cumulative ranking
curve (SUCRA) [12]. The SUCRA value was 100% when a treatment is
certain to be the best for efficacy but the worst for safety.
RESULTS
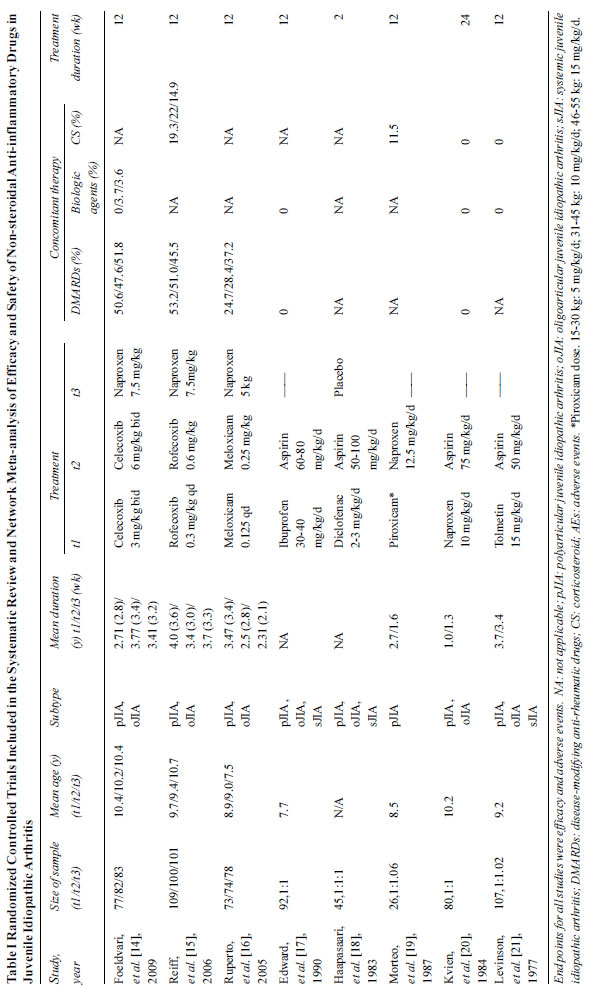
The selection of studies is shown in the
flowchart (Fig. 1). Overall, 8 studies provided data of
1112 individual JIA patients receiving the following NSAIDs:
celecoxib, rofecoxib, meloxicam, diclofenac, ibuprofen,
naproxen, piroxicam, and tolmetin. The study sample size ranged
from 26 to 310. The duration of treatments was from 2 weeks to
24 weeks. It was reported that there was no significant
difference in age, sex, course of disease between the groups.
The subtype of JIA contained poly-articular JIA, oligoarticular
JIA, and systemic JIA. The details of each included trial were
listed in Table I. Risk of bias within individual studies
was assessed (Fig. 2).
 |
|
Fig. 1 PRISMA 2009 flow diagram.
|
 |
|
Fig. 2 Risk of bias assessment.
|
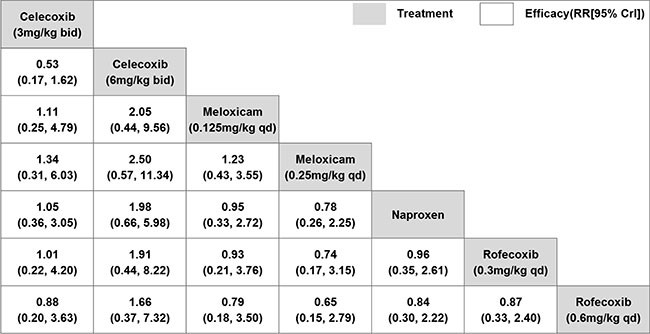
Network meta-analysis for efficacy:
Network meta-analysis was only performed when studies were
sufficiently homogeneous regarding outcome criteria. Thus, for
efficacy, three studies [14-16] with the efficacy criteria of
achieving an American College of Rheumatology Pediatric-30 (ACR
Pedi 30) response [22-23] were eligible. Four NSAIDs (celecoxib,
rofecoxib, meloxicam, and naproxen) were compared with at least
one other active drug directly and indirectly. There were no
significant differences between any two NSAIDs regarding
efficacy (Fig. 3). The ranking of treatments based on
cumulative probability plots and SUCRAs is shown in Web
Fig. 1. In terms of efficacy, celecoxib (6 mg/kg bid) had
the highest probability of being most effective (SUCRA = 76.4%),
while two doses of meloxicam ranked last.
 |
|
Fig. 3 Network meta-analysis of
efficacy of non-steroidal anti-inflammatory drugs for
juvenile idiopathic arthritis.
|
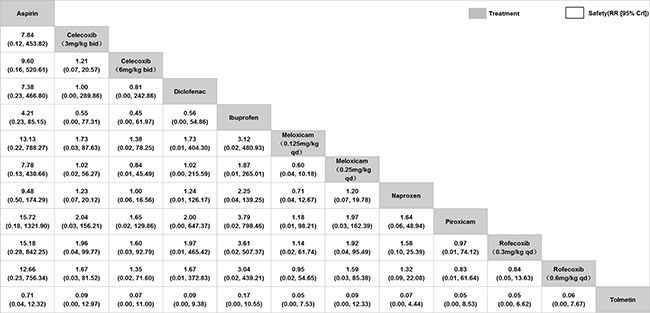
Network meta-analysis of the safety: Nine
NSAIDs (celecoxib, rofecoxib, meloxicam, naproxen, ibuprofen,
aspirin, diclofenac, piroxicam, and tolmetin) were directly
compared with at least one other active drug. There were no
significant differences between any two NSAIDs regarding safety
(Fig. 4). Ranking probability based on SUCRA
values indicated that rofecoxib (0.3 mg/kg/d) had the highest
probability of being the safest treatment (SUCRA=33.0%),
followed by piroxicam (SUCRA =35.5%). Tolmetin and aspirin
appeared to have the worst safety probability (SUCRA=82.3% and
82.0%, respectively) (Web Fig. 2).
 |
|
Fig. 4 Network meta-analysis of
safety of non-steroidal anti-inflammatory drugs for
juvenile idiopathic arthritis.
|
Inconsistency plots assessing network
inconsis-tencies between direct and indirect estimates showed a
low possibility of inconsistencies that might significantly
affect the results. In addition, the results of the random and
fixed-effects models yielded the same interpretation, indicating
that the results were robust.
DISCUSSION
We conducted a network meta-analysis of
currently available literature regarding NSAIDs for children and
adolescents with JIA. Unlike previous meta-analyses, we were
able to generate a ranking order for the relative efficacy and
safety of NSAIDs in patients with JIA. We found that the rate of
efficacy observed in all treatment groups in our study were
above the pooled composite placebo response rate (28.9%)
reported in a meta-analysis of six placebo-controlled trials
[25]. However, no statistically significant differences were
observed between NSAIDs in terms of efficacy or safety. The
findings are similar to the previous meta-analysis on NSAIDs for
osteoarthritis in adults [26,27]. The SUCRA ranking suggests
that celecoxib had better efficacy, while piroxicam and
rofecoxib have higher safety probabilities, compared to other
NSAIDs.
The most common adverse effects across all
treatment groups were gastrointestinal side effects, rash,
headache, and pyrexia. These side effects occurred more
frequently within the aspirin, tolmetin, and ibuprofen groups,
resulting in more non-compliance. Estimates of NSAIDs-associated
gastropathy range from 0.7-75%, depending on different study
designs [28-32]. Most of the gastrointestinal disorders were
mild, while serious gastropathy such as gastrointestinal
perforation and massive gastrointestinal hemorrhage was lower
than adults. The combination of glucocorticoid, leflunomide, and
methotrexate can aggravate gastrointestinal adverse reactions.
While children have a very low risk of cardiovascular
thromboembolic and serious gastro-intestinal events, prolonged
use of NSAIDS into adulthood could make them vulnerable to such
risks, especially when associated with other risk factors such
as obesity or smoking [36].
We used the GRADE (Grading of Recommendations
Assessment, Development, and Evaluation) approach to assess the
quality of the evidence related to our outcomes. The eight
included studies themselves were of moderate quality; however,
there may be circumstances where the overall rating for a
particular outcome would need to be adjusted per GRADE
guidelines [37]. The sample size for some comparisons was
assessed as a high bias of risk, which largely restricts the
quality of meta-analysis. Among the included studies, there were
no two studies that investigated the same type of NSAID compared
with another type of NSAID, which might overestimate the
efficacy and safety of treatments. Additionally, the
inconsistent criteria of efficacy make it impossible to make a
comprehensive comparison of some NSAIDs. Also, there was no data
on the stratification of subtypes and concomitant therapy, thus
it is unlikely to eliminate the impact of these factors on
efficacy and safety. The follow-up time points were limited from
only 2 to 24 weeks. The quality of the evidence (GRADE rating)
for the efficacy and safety of NSAIDs is very low, meaning there
is no evidence to support or refute the findings.
In conclusion, this Bayesian network
meta-analysis involving eight RCTs with low quality of evidence
showed that, in terms of efficacy, celecoxib (6 mg/kg bid)
ranked best among the four NSAIDs (celecoxib, rofecoxib,
meloxicam, and naproxen). In terms of safety, rofecoxib,
piroxicam, and meloxicam may be better than others. However, the
limitations of the study and suboptimal quality of evidence bar
us from making strong conclusions about the comparative efficacy
or safety of NSAIDs used to treat JIA. Further well-designed
RCTs are needed to figure out the best NSAID for JIA.
Acknowledgments: Mao Song for
technical assistance and Xiao-hui Tan for language editing.
Contributers: TX: conceived and
designed the study, critically revised the manuscript;
SC: acquired data, interpreted data, and drafted and critically
revised the manuscript. ZY, ZZ, ZJ: critically revised the
manuscript; SC, TX: screened and selected articles; SC, ZY, TX:
assessed the quality of included trials. All the authors read
and approved the final manuscript.
Competing interests: None stated.
Funding: None.
REFERENCES
1. Ross EP, Taunton RS, Prudence M, et al.
International League of Associations for Rheumatology
Classification of Juvenile Idiopathic Arthritis: Second
Revision, Edmonton, 2001. J Rheumatol. 2004;31:390-2.
2. Sarah R, Sheila TA, Timothy B, et al..
2019 American College of Rheumatology/Arthritis Foundation
Guideline for the Treatment of Juvenile Idiopathic Arthritis:
Therapeutic Approaches for Non-Systemic Polyarthritis,
Sacroiliitis, and Enthesitis. Arthritis Rheumatol.
2019;71:846-63.
3. Andrew RM, Sheena D, Geoffrey TM, Henry
JM. Tolerability and adverse events in clinical trials of
celecoxib in osteoarthritis and rheumatoid arthritis: Systematic
review and meta-analysis of information from company clinical
trial reports. Arthritis Res Ther. 2005;7:644-65.
4. Hetao H, Jianke P, Weiyi Y, et al.
Celecoxib vs diclofenac sodium in patients with knee
osteoarthritis: A protocol for systematic review and
meta-analysis. Medicine (Balti-more). 2020,99:e19680.
5. Ruijie W, Pin L, Heng J. The efficacy of
celecoxib for pain management of arthroscopy: A meta-analysis of
rando-mized controlled trials. Medicine (Baltimore). 2019;98:
e17808.
6. Deborah MC, Ades AE, Higgins JPT.
Simultaneous comparison of multiple treatments: Combining direct
and indirect evidence. BMJ. 2005;331:897-900.
7. Ferrán CL, Aurelio T, Chris C, David M,
Brian H. Network meta-analysis for comparing treatment effects
of multiple interventions: An introduction. Rheumatol Int.
2014;34: 1489-96.
8. Moher D, Liberati A, Tetzlaff J, Altman
DG. Preferred reporting items for systematic reviews and
meta-analyses: The PRISMA statement. BMJ. 2009;339:b2535.
9. Higgins JPT, Thomas J, Chandler J, et al.
Cochrane Handbook for Systematic Reviews of Interventions
version 6.0 (updated July 2019). Cochrane, 2019. Available from:
http://www.training.cochrane.org/ handbook.
10. Dechartres A, Altman DG, Trinquart L,
Boutron I, Ravaud P. Association between analytic strategy and
estimates of treatment outcomes in meta-analyses. JAMA.
2014;312: 623-30.
11. Shim S, Yoon BH, Shin IS, Bae JM. Network
meta-analysis: Application and practice using Stata. Epidemiol
Health. 2017;39:e2017047.
12. Salanti G, Ades AE, Ioannidis JPA.
Graphical methods and numerical summaries for presenting results
from multiple-treatment meta-analysis: An overview and tutorial.
J Clin Epidemiol. 2011;64:163-71.
13. Bhettay E, Thomson AJ. Double-blind study
of ketoprofen and indomethacin in juvenile chronic arthritis. S
Afr Med J. 1978;54:276-8.
14. Foeldvari I, Szer IS, Zemel LS, et al. A
prospective study comparing celecoxib with naproxen in children
with juvenile rheumatoid arthritis. J Rheumatol. 2009;36:174-82.
15. Reiff A, Lovell DJ, Adelsberg JV, et al.
Evaluation of the comparative efficacy and tolerability of
rofecoxib and naproxen in children and adolescents with juvenile
rheumatoid arthritis: A 12-week randomized controlled clinical
trial with a 52-week open-label extension. J Rheumatol.
2006;33:985-95.
16. Ruperto N, Nikishina I, Pachanov ED, et
al. A randomized, double-blind clinical trial of two doses of
meloxicam compared with naproxen in children with juvenile
idiopathic arthritis: Short- and long-term efficacy and safety
results. Arthritis Rheum. 2005;52:563-72.
17. Giannini EH, Brewer EJ, Miller ML, et al.
Ibuprofen suspension in the treatment of juvenile rheumatoid
arthritis. Pediatric Rheumatology Collaborative Study Group. J
Pediatr. 1990;117:645-52.
18. Haapasaari J, Wuolijoki E, Ylijoki H.
Treatment of juvenile rheumatoid arthritis with diclofenac
sodium. Scand J Rheumatol. 1983;12:325-30.
19. García MO, Maldonado CJA, Cuttica R,
Garay SM. Piroxicam in juvenile rheumatoid arthritis. Eur J
Rheumatol Inflamm. 1987;8:49-53.
20. Kvien TK, Høyeraal HM, Sandstad B.
Naproxen and acetylsalicylic acid in the treatment of
pauciarticular and polyarticular juvenile rheumatoid arthritis.
Assessment of tolerance and efficacy in a single-centre 24-week
double-blind parallel study. Scand J Rheumatol. 1984;13:342-50.
21. Levinson JE, Baum J, Brewer E, et al.
Comparison of tolmetin sodium and aspirin in the treatment of
juvenile rheumatoid arthritis. J Pediatr. 1977;91:99-804.
22. Giannini EH, Ruperto N, Ravelli A, Lovell
DJ, Felson DT, Martini A. Preliminary definition of improvement
in juvenile arthritis. Arthritis Rheum. 1997;40:1202-9.
23. Burnett HF, Regier DA, Feldman BM, Miller
FA, Ungar WJ. Parents’ preferences for drug treatments in
juvenile idiopathic arthritis: A discrete choice experiment.
Arthritis Care Res (Hoboken). 2012;64:1382-91.
24. Demirkaya E, Ruperto N , Galasso R. A
meta-analysis to estimate the "real" placebo effect in juvenile
idiopathic arthritis (JIA) trials. Pediatr Rheumatol.
2011;9:192-92.
25. Song GG, Seo YH, Kim JH, Choi SJ, Lee YH.
Relative efficacy and tolerability of etoricoxib, celecoxib, and
naproxen in the treatment of osteoarthritis: A Bayesian network
meta-analysis of randomized controlled trials based on patient
withdrawal. Z Rheumatol. 2016;75:08-16.
26. Castellsague J, Riera GN, Calingaert B,
Lorenzo CV. Individual NSAIDs and upper gastrointestinal
compli-cations: A systematic review and meta-analysis of
obser-vational studies (the SOS project). Drug Saf. 2012;35:
1127-46.
27. DeWitt EM, Sherry DD, Cron RQ. Pediatric
rheumatology for the adult rheumatologist I: Therapy and dosing
for pediatric rheumatic disorders. J Clin Rheumatol. 2005;11:
21-33.
28. Keenan GF, Giannini EH, Athreya BH.
Clinically significant gastropathy associated with nonsteroidal
antiinflammatory drug use in children with juvenile rheumatoid
arthritis. J Rheumatol. 1995;22:1149-51.
29. Mulberg AE, Linz C, Bern E, Tucker L,
Verhave M, Grand RJ. Identification of nonsteroidal
antiinflammatory drug-induced gastroduodenal injury in children
with juvenile rheumatoid arthritis. J Pediatr. 1993;122:647-9.
30. Hermaszewski R, Hayllar J, Woo P.
Gastro-duodenal damage due to non-steroidal anti-inflammatory
drugs in children. Br J Rheumatol. 1993;32:69-72.
31. Dowd JE, Cimaz R, Fink CW. Nonsteroidal
anti-inflammatory drug-induced gastroduodenal injury in
children. Arthritis Rheum. 1995;38:1225-31.
32. Bertagnolli MM, Eagle CJ, Zauber AG, et
al. Five-year efficacy and safety analysis of the adenoma
prevention with celecoxib trial. Cancer Prev Res (Phila).
200;2:310-21.
33. Bresalier RS, Sandler RS, Quan H, Baron
JA. Cardio-vascular events associated with rofecoxib in a
colorectal adenoma chemoprevention trial. N Engl J Med.
2005;352: 1092-102.
34. Muntner P, He J, Cutler JA. Trends in
blood pressure among children and adolescents. JAMA.
2004;291:2107-13.
35. Juonala M, Järvisalo MJ, Mäki TN, Kahoen
M, Viikara JS, Raitakari OT. Risk factors identified in
childhood and decreased carotid artery elasticity in adulthood:
The cardiovascular risk in young finns study. Circulation. 2005;
112:1486-93.
36. Guyatt G, Oxman AD, Sultan S, et al.
GRADE guidelines: 11. Making an Overall Rating of Confidence in
Effect Estimates for a Single Outcome and for All Outcomes. J
Clin Epidemiol. 2013;66:151-7.
|
|
|
 |
|

