|
|
|
Indian Pediatr 2021;58:144-148 |
 |
Extended-Spectrum
b-Lactamase-Producing
Enterobacteriaceae Causing Community-Acquired Urinary Tract
Infections in Children in Colombia
|
|
Jhon Camacho-Cruz, 1
Javier Munoz Martinez,1
Julio Mahecha Cufino,1
German Camacho Moreno,1
Carolina Rivera Murillo,1
Maria Alejandra Suarez Fuentes,1
and Carlos Alberto Castro2
From Departments of 1Pediatrics and 2Medical Epidemiology, FundaciónUniversitaria de Ciencias de la Salud (FUCS) – Hospital de San
José and Hospital Infantil Universitario de San José de Bogotá,
Colombia.
Correspondence to: DrJhon Camacho, Associate Instructor,
Department of Pediatrics FUCS,Calle 10, No.18-75 Bogota, Colombia.
Email:
[email protected]
Received: March 24, 2020;
Initial review: April 29, 2020;
Accepted: December 03, 2020.
|
Objective: To characterize the pediatric patients presenting at the
two pediatric centers in Bogotá, with first isolate urine culture of
community-acquired extended-spectrum
b-lactamase
(ESBL)-producing enterobacteriaceae. Methods: Review of
microbiological data of children between January, 2012 and December,
2018, obtained using the WHONET software. Results: A total
of 2657 Escherichia coli, Klebsiella spp and Proteus mirabilis -
positive urine cultures were obtained within a 6-year period; data of
132 patients were finally selected. Frequency of
ESBL-producing bacteria infections in community-acquired urinary tract
infections (UTI) was 5%: 123 E. coli (93.2%), 7 K. pneumoniae
(5.2%), 1 K. oxytoca (0.8%), and 1 P. mirabilis (0.8%).
Conclusion: A predominance of female sex, preschool children, and
lower tract urinary infections were found, as well as a low frequency of
comorbidities. Adequate sensitivity to amikacin and nitrofurantoin was
found in this study.
Keywords: Escherichia coli, Klebsiella spp, Management,
Prevalence, Sensitivity.
|
|
G
ram-negative bacteria are a common cause
of urinary tract infections (UTIs) in children, and are
frequently being reported as extended-spectrum
b-lactamase
(ESBL)-producing bacteria [1]. Actual incidence of urinary
infections due to ESBL-producing bacteria in children is
difficult to estimate; however, over the last 10 years,
resistance has been gradually increasing around the world [2].
Adult studies report a prevalence of 3% to
16.3% among all UTI patients [1], whereas a prevalence of 10.9%
has been reported in a pediatric study [3]. There are few
pediatric studies, estimating the prevalence and the incidence
of ESBL-producing enterobacteriaceae in community-acquired UTIs.
We report the frequency and the clinical characteristics of
children presenting with urinary infections and a urine culture
with community-acquired ESBL-producing bacteria in two hospitals
of Bogotá.
METHODS
A hospital record review of patients younger
than 18 years was done from the emergency department of Hospital
de San José and Hospital Infantil Universitario de San José in
Bogotá. Children included were those with urinary symptoms or
febrile condition without focus and initially an ESBL-producing
bacteria was isolated for the first time in the urine culture
from January, 2012 to December, 2018. Those with positive urine
cultures for healthcare-associated infections (defined as
hospital-acquired infections during treatment or care for a
medical condition) and reinfections (two or more UTI by
ESBL-producing bacteria) were excluded. Socio-demographic and
clinical variables were evaluated, including additional
diagnoses, underlying pathologies, inpatient manage-ment, and
characteristics related to antibiotic treatment. Information was
collected using an instrument developed by the investigators,
which was completed based on the review and selection of medical
records that met the inclusion criteria. Microbiological
information was obtained using the WHONET 5.6 software (World
Health Organization). Minimum inhibitory concentrations (MIC)
were also rated and interpreted in accordance with the
recommendations of the Clinical and Laboratory Standards
Institute (CLSI) of 2017 [4], measured based on the MicroScan
(Baxter) parameters in Hospital San José and on the VITEK2 (bioMerieux)
automated method, in the Hospital Infantil Universitario de San
José.
Statistical analyses: Information was
stored in a database to be then validated selecting 10% of the
records, and compared against the instruments. A descriptive
analysis of the information was conducted in STATA 12;
qualitative variables were presented with absolute and relative
frequencies, while quantitative variables included central
tendency and scatter measurements, in accordance with the
distribution of the data. This study was submitted and approved
by the ethics and the research on humans committees in both
hospitals.
RESULTS
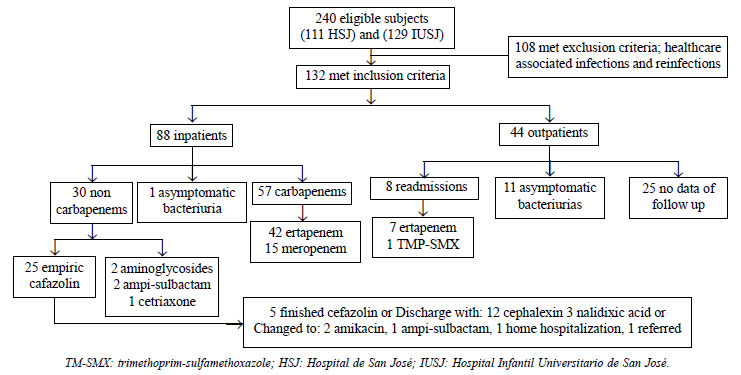
A total of 2657 positive urine cultures were
obtained, of which 240 were reported as ESBL-producing bacteria;
the medical records were reviewed, and in the end, 132 (81.8%
girls) patients were eligible for the final analysis (Fig.
1). Frequency of community-acquired ESBL-producing bacteria
isolates in first UTI was 5%. Of the 132 community-acquired
ESBL-producing bacteria isolates, 123 (93.2%) were from E.
coli, 7 (5.2%) from K. pneumoniae, and 1 (0.8%) each
from K. oxytoca, and Proteus mirabilis.
 |
|
Fig. 1 Flowchart of the
study.
|
The median (IQR) age of girls and boys age 4
(1-6.5) year and 0.5 (0.2-1) year, respectively. Other
character-istics of the population are shown in Table I.
Table I Clinical and Demographic Characteristics of Children With First Episode of Urinary
Tract Infection by Community-Acquired ESBL-Producing Enterobacteriaceae (N=132)
| Characteristics |
No. (%) |
| Age (mo) |
|
| <12 |
28 (21.2) |
| 13-24 |
22 (16.7) |
| 25-60 |
45 (34.1) |
| 61-144 |
30 (22.7) |
| >145 |
7 (5.3) |
| Comorbidities |
|
|
Renal and urological comorbiditiesa |
18 (13.6) |
|
Other comorbiditiesb
|
15 (11.4) |
| Diagnosis |
|
|
Asymptomatic bacteriuria |
12 (9.1) |
| Lower UTI |
102 (77.3) |
| Upper UTI |
18 (13.6) |
|
History of hospitalizationc |
28 (21.2) |
|
History of surgeryc |
8 (6.1) |
|
Previous antibiotic therapyc |
50 (37.9) |
| UTId |
49 (37.1) |
|
Congenital malformatione |
26 (19.7) |
| Hospital stay (d), median (IQR)
|
9 (6-11) |
| UTI: Urinary tract
infection; ESBL; Extended spectrum beta lacta-mase;
aRenal and urological comorbidities: hydronephrosis,
pyelectasis, horseshoe kidney, nephrotic syndrome,
bladder diverticula, vesicoureteral
reflux, kidney transplant and nephrostomy; bOther
comorbidities: myelomeningocele, anemia,
chromosomo-pathy, cholestasis, hydrocephalus, systemic
lupus erythematous, pulmonary hypertension. c3 months
prior to ED visit; ddue to ESBL non-producing germs;
epredisposing to UTI. |
Additionally, 49 patients (37.1%) had a
previous history of UTIs, 25 patients (18.9%) had UTI-associated
congenital malformations, 19 (76%) had renal or urologic
conditions (hydronephrosis (n=9), pyelectasis (n=5),
horseshoe kidney (n=2), renal hypoplasia (n=1),
duplex collector system (n=1) and renal duplication (n=1)
and 6 patients (24%) presented with neurological malfor-mations
(myelomeningocele (n=5), hydrocephalus (n=1).
Among this group of malformations, 18 (69.2%) presented a
history of previous UTI. Furthermore, four patients (3.0%) were
in immunosuppressive therapy: two cases of nephrotic syndrome,
one case of systemic lupus erythe-matous and one due to chronic
kidney failure. Nine patients (6.8%) exhibited an additional
risk because of self-medication with amoxicillin, cephalexin,
trimethoprim sulfamethoxazole (TMP-SMX) or metronidazole. 50
patients (37.9%) had no relevant history or risk factors for
UTI.
Imaging findings showed 92 (69.6%) patients
undergoing kidney and urinary tract ultrasound, of which 52
(56.5%) had normal results, 13 (14.1%) exhibited enlarged
kidneys, 9 (9.8%) had evidence of pyelocalicea-lectasia, 7
(7.6%) with kidney atrophy, 6 (6.5%) sediments in urine, 3
(3.2%) hydronephrosis, 1 (1.1%) duplicated pyelocaliceal system,
and 1 (1.1%) neurogenic bladder). Out of 24 patients undergoing
cystouretrography, 12 (50%) were normal, 7 (29.2%) had
vesicoureteral reflux, and 2 (8.3%) bladder diverticula; the
remaining three patients (12.5%) had penile hypospadias,
postvoid residual urine, and decreased posterior urethral
diameter. Finally, of 22 patients undergoing renal gammagraphy,
12 (54.5%) had documented pyelonephritis, 8 (36.4%) were normal,
and 2 (9.1) had kidney scarring.
Sensitivity profiles and co-resistance for
E. coli and K. pneumoniae are illustrated in Table
II. Supplementary Fig. 1 shows the
detailed MICs for E. coli for the most important
antibiotics, using automated methods and their relationship to
the CLSI to define sensitivity and resistance.
Table II Specific Sensitivity CLSI 2017 of Each Antibiotic According to the ESBL-Producing
Bacteria Isolated in Positive Urine Culture (N=130)a
|
Escherichia coli
|
Klebsiella pneumoniae |
| Antibiotic |
Sensitive |
Intermediate |
Resistant |
Sensitive |
Intermediate |
Resistant |
|
Ampicillin sulbactam, n=130 |
46 (35.4) |
25(19.2) |
52 (40) |
3 (2.3) |
0 |
4 (3.1) |
| Piperacillin-tazobactam, n=68 |
56 (82.4) |
3 (4.4) |
5 (7.4) |
4 (5.8) |
0 |
0 |
| Amikacin, n=130 |
121 (93) |
1 (0.8) |
1 (0.8) |
7 (5.4) |
0 |
0 |
| Gentamicin, n=130 |
88 (67.7) |
1 (0.8) |
34 (26.1) |
6 (4.6) |
0 |
1 (0.8) |
| TMP-SMX, n=128 |
33 (25.8) |
0 (0.0) |
88 (68.8) |
3 (2.3) |
0 |
4 (3.1) |
| Nitrofurantoin, n=129 |
92 (71.3) |
28 (21.7) |
2 (1.5) |
5 (3.9) |
1 (0.8) |
1 (0.8) |
| Fosfomycin, n=57 |
52 (91.3) |
0 |
1 (1.7) |
3 (5.3) |
0 |
1 (1.7) |
| Ciprofloxacin, n=129 |
59 (45.7) |
2 (1.6) |
61 (47.3) |
4 (3.1) |
0 |
3 (2.3) |
| Meropenem, n=130 |
130 (100) |
0 |
0 |
130 (100) |
0 |
0 |
|
aOne case each of P. mirabilis and
K.oxytoca were excluded; Values in no. (%); all n not
equal to 130 because not all isolates had the disks for
that antibiotic in the antibiogram. |
After the initial assessment 44 patients,
empiric outpatient therapy was administered with cephalexin in
39 cases (88.7%), nalidixic acid in 2 cases (4.5%), no
antibiotic therapy was prescribed in 2 cases (4.5%), while
TMP-SMX was used in 1 case (2.3). Of these patients, 8 (18.2%)
relapsed and were admitted for ertapenem treatment (7) and 1 was
discharged with TMP-SMX. The remaining 36 patients (81.8%); 11
were considered to develop asymptomatic bacteriuria and 25
patients had no outpatient follow-up information available.
88 patients received hospitalized management,
70 (79.5%) were initially treated with cephalosporins, 7 (7.9%)
with aminoglycosides, and 11 (12.6%) received other antibiotic
therapies. Once the results of the urine culture were available,
57 (64.8%), received specific therapy with carbapenems:
ertapenem (n=42) and mero-penem (n=15); 31
patients (35.2%) received another betalactamic antibiotic
therapy (n=28) and aminogly-cosides (n=3).
Of the 107 patients followed, 12 were
considered asymptomatic bacteriurias, and 95 received empirical
treatment. Of these 95 patients, 74 (77.8%) required switching
over to carbapem management and 21 (22.2%) patients experienced
no change in their antibiotic treatment.
In terms of outcomes, one 7-month old patient
died, with Down syndrome admitted with a diagnosis of upper UTI
and E. coli isolate. The patient received empirical
treatment with ampicillin sulbactam, which was switched on day
three to ertapenem, based on the urinary culture results.
However, the condition of the patient progressed to septic
shock.
Hospitalization in last three months (n=28,
21.2%), recurrent urinary tract infection (n=49, 37.1%),
previous use of antibiotics (n=50, 37.9%) and urinary
tract abnormalities were findings for ESBL producing
community-acquired UTI.
DISCUSSION
This study presents the frequency of UTI
associated with community acquired ESBL-producing bacteria
similar to the levels reported in the international literature
[1,5]. Demographic characteristics include a higher frequency of
females, with an age group distribution similar to the reported
in the world literature [6,7]. The factors related with
ESBL-producing bacteria UTIs are: a history of a previous UTI
non ESBL-producing bacteria, urinary tract malformations,
hospitalization, or antibiotic therapy during the last 3 months
(40% first generation cephalos-porins) [5,8,9]. In terms of
comorbidities, surprisingly most patients were previously
healthy (without any comorbidities), which correlates with the
circulation of ESBL-producing enterobacteriaceae phenotype in
the community.
Median for hospital days is high, similar to
previous studies [10], in addition to a more than two-fold
increase in costs [9,11]. This may be due to the fact that
patients with infections from ESBL-producing bacteria have a
higher risk of hospitalization because of their past history
[6]. Findings of imaging studies were mostly normal and among
the most frequent ultrasound alterations were enlarged kidneys,
followed by pyelectasis; the number of patients who underwent
cystouretrographies and renal gammagraphies was low in contrast
with literature [6] as for most in our population it was their
first infection [12].
The most commonly isolated bacteria was E.
coli, so a more detailed analysis was performed of the
resistance to 8 antibiotics. A very high sensitivity was found
to fosfomycin, nitrofurantoin and amikacin, with similar
findings to those in the spanish study by Pérez, et al. [1]. A
variable sensitivity and resistance was also identified in the
group of betalactamase inhibitors, with a higher resistance in
the ampicillin sulbactam group and intermediate sensitivity,
with MIC approaching the resistance to piperacillin-tazobactam.
It is therefore hypothesized that these are not sound
therapeutic options in this scenario, because of the risk if
increased resistance as has been stated by other authors
[11,13]. A proportion of inpatients were treated with initial
empirical management with cephazolin, achieving a satisfactory
clinical evolution with a negative control urine culture. The
correlation of urinary concentrations that the drug can reach
should be studied [14]. In this series of patients, the typical
risk factors described in the literature were uncommonly seen
[7,15].
It can be suggested that this pathology may
be underdiagnosed or even treated incorrectly, impacting on
bacterial resistance and as a result in prognosis; forcing
health professionals treating this disease to explore the
presence of ESBL in a non-hospital population and without the
risk factors specified in the literature. Other studies should
be done to confirm if there are strains of multi-resistant
bacteria circulating in the community.
Taking into account the observational nature
of this study and the retrospective collection of data, it is
not possible to determine causality. However, this drawback was
offset using different sources of information. Further
analytical and experimental studies are needed to validate the
hypotheses herein discussed and propose an analytical study to
confirm if there are strains of multi-resistant bacteria
circulating in the community. Another limitation of study was
that discs of antibiotic for antibiogram were not uniformly
available for all cases.
UTIs from community acquired ESBL-producing
enterobacteriaceae are a serious public health issue as a result
of the increasing number of cases over the last decade. This
population presents a frequency of 5% for E. Coli and
K. pneumoniae. A predominance of low urinary infection was
found in previously healthy girls of preschool age. The typical
risk factors associated with ESBL-producing bacteria infections
in community acquired UTI were low in this population. This
study reveals the epidemiological and microbiological profile of
these hospitals, good sensitivity was found in this population
for amikacin and nitrofurantoin, so as to select an adequate
treatment and to design alternative non-carbapenem antibiotic
protocols for outpatients, with a view to promote the rational
use of antibiotics. Fosfomycin, piperacillintazobactam, and
other antibiotics require further investigation.
Ethics Clearance: Ethics and the research
on humans committees in both hospitals. Hospital ethics review
board (Comité de ética en investigación con sereshumanos
Hospital de San José, CEISH-HSJ). No. 0369-2018, dated September
17, 2018.
Acknowledgments: Diana Ortiz,
microbiologist of Hospital San Jose and Sandra Peña,
Head,Clinical Laboratory, Hospital InfantilUniversitario de San
José, for their contribution to the microbiological data.
Special thanks to the research division of
FundaciónUniversitaria de Ciencias de la Salud, Hospital de San
José, Bogotá, Colombia, for the translation of the manuscript.
Dr. Pablo VásquezHoyos for his guidance on methodology, and Dr.
Adriana Jiménez for sharing the data used for this article.
Contributors: JCC, JMM, JMC:
conceptualized and designed the study, drafted the initial
manuscript, and reviewed and revised the manuscript; GCM, CRM,
MASF, CCM: designed the data collection instruments, collected
data, carried out the initial analyses, and reviewed and revised
the manuscript. All authors critically reviewed the manuscript
for important intellectual content, approved the final
manuscript as submitted, and agree to be accountable for all
aspects of the work.
Funding: Fundación Universitaria de
Ciencias de la Salud (internal support for academic research
projects).
Competing interests: None stated.
REFERENCES
1. Perez Heras I, Sanchez-Gomez JC, Beneyto-Martin
P, Ruano-de-Pablo L, Losada-Pinedo B. Community-onset
extended-spectrum beta-lactamase producing Escherichia coli in
urinary tract infections in children from 2015 to 2016:
Prevalence, risk factors, and resistances. Medicine (Baltimore).
2017;96:e8571.
2. Fan N-C, Chen H-H, Chen C-L, et al. Rise
of community-onset urinary tract infection caused by
extended-spectrum b-lactamase-producing
Escherichia coli in children. Journal of Microbiology,
Immunology and Infection. 2014;47:399-405.
3. Jurado L, Camacho G, Leal A, et al.
Clinical, phenotypic and genetic characterization of
extended-spectrum beta-lactamase-producing Enterobacteriaceae (E.
coli, K. pneumoniae and Proteus spp.) in
community-acquired urinary tract infections [Article in
Spanish]. Infectio: X Encuentro Nacional de Investigadores en
Enfermedades Infecciosas, Medellín 2016.p.24.
4. Wayne P. Performance Standars for
Antimicrobial Susceptibility Testing. 27th ed: Clinical and
Laboratory Standards Institute; 2017.
5. Kim YH, Yang EM, Kim CJ. Urinary tract
infection caused by community-acquired extended-spectrum
b-lactamase-producing
bacteria in infants. J Pediatr (Rio J). 2017;93: 260-266.
6. Kizilca O, Siraneci R, Yilmaz A, et al.
Risk factors for community-acquired urinary tract infection
caused by ESBL-producing bacteria in children. Pediatr Int.
2012;54:858-62.
7. Topaloglu R, Er I, Dogan BG, et al. Risk
factors in community-acquired urinary tract infections caused by
ESBL-producing bacteria in children. Pediatr Nephrol.
2010;25:919-25.
8. Rodríguez-Baño J, Pascual A. Clinical
significance of extended-spectrum
b-lactamases.
Expert Review of Anti-infective Therapy. 2008;6:671-83.
9. Dayan N, Dabbah H, Weissman I, Aga I, Even
L, Glikman D. Urinary tract infections caused by
community-acquired extended-spectrum
b-lactamase-producing
and non-producing bacteria: A comparative study. J Pediatri.
2013;163: 1417-21.
10. Nieminen O, Korppi M, Helminen M.
Healthcare costs doubled when children had urinary tract
infections caused by extended-spectrum beta-lactamase-producing
bacteria. Acta Paediatr. 2017;106:327-33.
11. Sheu CC, Lin SY, Chang YT, Lee CY, Chen
YH, Hsueh PR. Management of infections caused by
extended-spectrum beta-lactamase-producing Enterobacteriaceae:
Current evidence and future prospects. Expert Rev Anti Infect
Ther. 2018;16:205-18.
12. Sundar S, Chinnasami B, Sadasivam K,
Pasupathy S. Role of imaging in children with urinary tract
infections. Int J Contemp Pediatr. 2017;4:751-55.
13. Tamma PD, Rodriguez-Bano J. The use of
non-carbapenem b-lactams
for the treatment of extended-spectrum
b-lactamase
infections. Clin Infect Dis. 2017;64: 972-80.
14. Wang KC, Liu MF, Lin CF, Shi ZY. The
impact of revised CLSI cefazolin breakpoints on the clinical
outcomes of Escherichia coli bacteremia. J Microbiol Immunol
Infect. 2016;49:768-774.
15. Balasubramanian S, Kuppuswamy D,
Padmanabhan S, Chandramohan V, Amperayani S. Extended-spectrum
beta-lactamase-producing community-acquired urinary tract
infections in children: Chart review of risk factors. J Glob
Infect Dis. 2018;10:222-25.
|
|
|
 |
|

