|
|
|
Indian Pediatr 2019;56:126-129 |
 |
Capillary versus
Serum b-hydroxybutyrate
in Pediatric Diabetic Ketoacidosis
|
|
Praveen M Kurup 1,
Ramachandran Rameshkumar1,
Rajendran Soundravally2
and Ponnarmeni Satheesh1
From Departments of 1Pediatrics and
2Biochemistry, Jawaharlal Institute of Postgraduate Medical
Education and Research (JIPMER), Puducherry, India.
Correspondence to: Dr Rameshkumar R, Associate
Professor, Division of Pediatric Critical care, Department of
Pediatrics, (JIPMER), Puducherry 605 006, India .
Email:
[email protected]
Received: March 29, 2018;
Initial review: August 20, 2018;
Accepted: November 21, 2018.
Trial registration: Clinical Trial Registry of
India (CTRI/2017/05/008690).
|
Objective: To find the strength of
agreement between point-of-care and serum
b-hydroxybutyrate.
Methods: 236 paired samples (capillary
b-hydroxybutyrate
by a point of care device and serum
b-hydroxybutyrate by colorimetric
enzymatic estimation) samples were collected from 26 children aged <13
years admitted with diabetic ketoacidosis. Inborn errors of metabolism
and septic shock were excluded. Results: Capillary
b-hydroxybutyrate
showed excellent agreement with serum â-hydroxybutyrate with mean (SD)
bias of 0.027 (0.78); 95% limit of agreement -1.51, 1.56 and intraclass
correlation 96.1% (95%CI 95%–97%, P<0.001). An
increase in the bias noted for value above 5 mmol/L (P<0.001)
(serum measurements were higher than capillary point-of-care measure-ments).
Capillary â-hydroxybutyrate correlated significantly with blood pH,
anion gap,bicarbonate and carbon dioxide levels on blood gas analysis (P<0.05).
Conclusions: Capillary b-hydroxybutyrate
estimation is a valid method for monitoring of ketonemia in pediatric
diabetic ketoacidosis.
Keywords: Diagnosis, Ketonemia, Point-of-care, Type-I diabetes
mellitus.
|
|
D
iabetic ketoacidosis (DKA) is
characterized
by the triad of hyperglycemia, acidosis, and
ketosis. Among blood ketones, beta-hydroxybutyrate (BOHB) predominates in DKA (acetoacetate: BOHB
increase up to 1:10 against the normal 1:1), which is the basis
of monitoring of blood BOHB in DKA. Current guidelines recommend
periodic monitoring of ketone bodies (blood/urine) [1]. Urine ketone
estimation, does not provide an accurate estimate of the ketone status
as it measures acetoacetate instead of BOHB, and often requires urinary
catheterization. Estimation of blood BOHB requires expensive equipment
and often fails to provide real-time results. Alternatively, the
point-of-care (POC) BOHB meter is a simple handheld device that can work
accurately both as capillary glucose and a BOHB sensor [2,3]. The
purpose of this study was to describe the strength of correlation and
agreement between capillary and serum BOBH and correlation with blood
gas parameters.
Methods
The prospective study was undertaken in the Pediatric
critical care division in a tertiary-care hospital in Puducherry from
July 2015 to July 2017. Approval was obtained from the Institute Ethics
Committee of Jawaharlal Nehru Institute of Postgraduate Medical
Education and Research.
All children aged <13 years with DKA, as per
International Society for Pediatric and Adolescent Diabetes (ISPAD) 2014
definition, were included after written informed consent from
parents/legal guardian [1]. Children with suspected/known inborn errors
of metabolism (IEM) or having septic shock were excluded. Glycated
hemoglobins (HbA1c), venous blood gas, capillary and serum BOHB, blood
glucose, serum electrolytes, and renal and liver function tests were
obtained at admission and repeated 2-hourly for first first 6 hours, and
4-hourly (or more) till resolution of DKA (except hemogram and HbA1c
done only at baseline). Capillary blood glucose was measured every
30-minute till resolution of DKA. 1 mL of serial blood samples for serum
BOHB analysis was centrifuged, serum extracted, and stored at -80 oC
until final analysis. Capillary BOHB measurement was carried out using
the point-of-care device (Abbott Optium-H ketone meter, Illinois,
USA) after calibration with the calibration stick
provided by the manufacturer. Cayman colorimetric enzymatic BOHB
estimation kits were used for measurement of serum BOHB [4]. Both
methods are based on the quantification of NADH generated during the
enzymatic conversion of BOHB to acetoacetate. A BOHB level of >3 mmol/L
was suggestive of ketonemia [1]. Analysis of serum electrolytes, renal
and liver function tests were done in the biochemistry laboratory using
Olympus AU 680 (Beckman Coulter, California, USA). Blood gas estimation
was done using the blood gas analyzer (Cobas b 221 Blood Gas Analyzer,
Roche Diagnostics, Switzerland).
The laboratory (reference) method has a coefficient
of variation (COV) of 0.98, and point-of-care method has a COV of 0.96
[2,5]. With the power of 95%, and an
a-error of 5%, the
minimum sample required (i.e., pairs of capillary and serum) was 235,
including 10% attrition for hemolysis and laboratory errors. Sample size
calculation was done using n-Master version 2.0 (CMC, Vellore, India).
Normality of data was checked with Kolmogorov-Smirnov Z test. Cost
comparison between POC and serum measurement was made using student t
test. Intra-class correlation (ICC) with 95% confidence interval (CI)
and Bland-Altman plot was used to test the agreement between capillary
and serum BOHB. Linear regression was used for evaluating the
correlation of capillary and serum BOHB; capillary BOHB with pH, PCO2,
HCO3, and AG. Two-tailed
tests were used and P-value <0.05 considered as statistically
significant. SPSS version 20.0 software and Epi Info™ 7 was used for
data analysis. The laboratory technician was blinded to capillary BOHB
values. The statistician was blinded till preparation of the first
draft.
Results
Forty-eight children with DKA were assessed for
eligibility (22 excluded IEM=2, missed=14, refused to participate=6), 26
patients were enrolled. A total of 236 pairs of samples were analyzed
(39 excluded, hemolysis =30, leaked=9). POC and serum BOHB (mmol/L)
value less than one were 55 vs. 73,
³1 <3 was 92 vs.
76, ³3 <5 was
47 vs. 47, and ³5
was 42 vs. 40. Twenty one cases of DKA was found to have
ketonemia (BOHB ³3)
by POC method with excellent agreement with the reference method (Kappa
value 0.752, P=<0.001, sensitivity 95.2%). Five cases of DKA were
missed by POC method of which one case was diagnosed by the reference
method. The baseline characteristics and laboratory parameters are
described in Table I.
TABLE I Baseline Characteristics of Children with Diabetic Ketoacidosis at Enrolment (N=26)
|
Variables |
Patients |
Variables |
Patients
|
|
Age, y |
8.1 (3.9) |
Serum electrolytes
|
|
|
Male: Female, n (%) |
7 (27): 19 (73) |
Sodium, mEq/L |
133.3 (6.9) |
|
New onset DKA, n (%) |
13 (50) |
Potassium, mEq/L |
4.2 (0.7) |
|
Patients with recurrent DKA (³2 episodes), n (%) |
12 (46) |
Chloride, mEq/L |
106 (8) |
|
Male: Female ratio among recurrent DKA, n (%) |
2 (17): 10 (83) |
Venous blood gas
|
|
|
Weight Z score |
-3.0 (2.1) |
pH |
7.1 (0.1) |
|
Height Z score |
-1.3 (1.7) |
pCO2, mm of Hg |
22 (6) |
|
Body mass index, kg/m2 |
13.05 (1.75) |
Bicarbonate, mEq/L |
6.7 (3.2) |
|
PRISM III score, median (IQR) |
12 (11-12) |
Mild DKA, n (%) |
4 (15) |
|
Modified Glasgow coma scale, median (IQR) |
15 (14-15) |
Moderate DKA, n (%) |
7 (27) |
|
Time to hospitalization after first symptom, d |
3 (2-7) |
Severe DKA, n (%) |
15 (58) |
|
Serum beta-hydroxybutyrate, mmol/L |
4.5 (2.4) |
Glycated hemoglobin (%) |
12.6 (2.1) |
|
Random blood sugar, mg/dL |
475 (99) |
|
|
|
Capillary beta-hydroxybutyrate, mmol/L |
4.8 (1.7) |
|
|
|
All data are presented as mean (SD) unless otherwise
specified. DKA: Diabetic-ketoacidosis,PRISM: Pediatric risk of
mortality |
The correlation between POC and serum BOHB showed R2
= 0.863, P=<0.001, with a beta-coefficient (slope of the
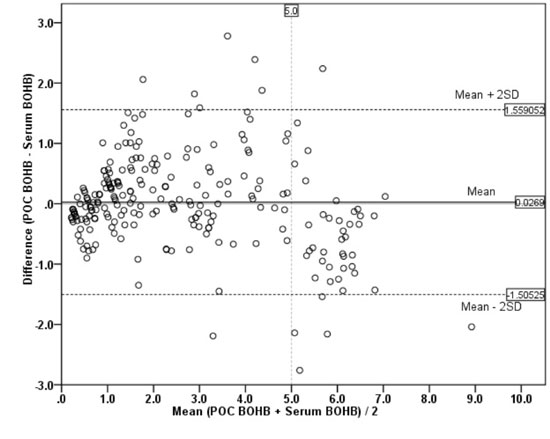
regression line) of 0.929 and intercept of 0.409. The Bland-Altman
analysis, as shown in Fig. 1, showed excellent agreement
with a mean (SD) bias of 0.027 (0.78) and 95% limit of agreement is 1.51
to -1.56. An increase in the bias was noted in values above 5 mmol/L (P<0.001)
(serum higher than POC). The intra-class correlation between POC and
serum BOHB was 96.1% (95% CI 95% to 97%, P<0.001). POC-BOHB
showed a moderate negative correlation with pH (r = -0.563, P<0.05)
and HCO3 (r = -0.557, P<0.05),
weak with pCO2 (r = -0.378,
P<0.05) and moderately positive with AG (r=0.478, P<0.05).
The mean (SD) total cost involved in POC-BOHB measurement per patient
was significantly lower as compared to laboratory method [`
1197 (402) vs. `
2903 (976); P<0.001]. This cost is exclusive of routine
investigations, equipment charges, workforce or other miscellaneous
costs. The mean (SD) time for resolution of DKA was 23.5 (13.2) hours.
 |
|
Fig. 1 Bland-Altman plot between
capillary and serum b-hydroxy
butyrate (BOHB) levels.
|
Discussion
In this study, we documented the excellent
correlation and agreement between POC and serum BOHB measurement with an
increase in the bias of value above five mmol/L. This can be due to
insufficient quantity of reagent on the electrochemical strip and
non-linearity of the ampero-metric detector in the device, and the
absolute quantity of acetoacetate may inhibit the enzyme in the test
strip or cause inhibition of the quinoid NADH redox mediator
incorporated into the electrode [3]. Studies are reported that excellent
agreement of POC measurement with standard measurement up to the value
of 4 mmol/L [6] and 6 mmol/L [3]. A BOHB value above 1.5 mmol/L
indicates that "at risk" for DKA and value
³3 mmol/L is a
diagnosis of DKA [1,6]. A BOHB value less than 1 mmol/L is one of the
endpoints of DKA treatment. The clinically relevant range of BOHB value
is <1-4 mmol/L [7]. Hence, POC device can be used as a reliable bedside
method of ketone (BOHB) estimation, provided that values above 5 mmol/L
be viewed with caution. In children, obtaining a urine sample is
impractical for ketone estimation, POC-BOHB has a unique value and also
can be utilized even in a primary healthcare set-up, as no new equipment
is required (the glucometer can be recalibrated as a ketone meter with
the help of a calibration stick).
The correlation of POC BOHB with blood gas parameters
has been the subject of evaluation in similar studies [6,8,9] except
lower correlation noted with bicarbonate, which is similar to our study
results. The findings of this study thus indicate that measurement of
serum BOHB can indicate the general trend of disease progression and
resolution. POC-BOHB has potential in obviating the need for blood gas.
However, if the value is above 5 mmol/L, blood gas analysis is still a
necessity. Newer generations of POC-BOHB meters with higher measurement
ranges may be able to solve this problem.
The study was limited by fewer samples with BOHB
value above 5 mmol/L, which makes it difficult to comment on the
accuracy of POC meters at high values. The findings of our study
indicate that future recommendation on the management of pediatric DKA
should include POC-BOHB monitoring as a convenient, cost-effective and
safe alternative to conventional blood gas estimation.
We conclude that the point of care capillary beta-hydroxybutyrate
estimation is as accurate as laboratory estimation and has a significant
correlation with blood sugar and blood gas parameters, thus making it a
reliable tool for monitoring of ketonemia in the management of pediatric
diabetic ketoacidosis.
Acknowledgments: We acknowledge the
contribution of Mrs. S. Raja Deepa (JIPMER Campus, Puducherry, India)
for review and editing of the manuscript; Mr. Rakesh Mohindra (Punjab
University, Chandigarh, India) and Miss. Thenmozhi M (CMC, Vellore,
India) for helping the statistical analysis and Miss. Harpreet Kaur
(Punjab University, Chandigarh, India), and Mrs. Neelima Chadha (Tulsi
Das Library, PGIMER, Chandigarh, India) for helping medical literature
search. They did not receive any compensation for their contributions.
Contributors: PMK, RR, PS: were involved in the
management of the patients; KP: collected the data, reviewed the
literature and drafted the first manuscript; PS: contributed for
protocol development, review of literature and manuscript; SR:
participated in protocol preparations and drafting of the manuscript and
supervised the analysis of biochemical samples; RR: conceptualized the
study, reviewed the literature and critically reviewed the manuscript.
All authors approved the final version of the manuscript; RR: is the
guarantor of the paper.
Funding: JIPMER intramural research grant
(JIP/Res/Intra-MD-MS/01/2015-16 to RR).
Competing interest: None stated.
|
What This Study Add?
•
Point of care capillary beta-hydroxybutyrate estimation device
has good correlation with laboratory beta-hydroxybutyrate
estimation, and offers a less costly way to monitor ketonemia in
pediatric diabetic ketoacidosis.
|
References
1. Wolfsdorf JI, Allgrove J, Craig ME, Edge J, Glaser
N, Jain V, et al. ISPAD Clinical Practice Consensus Guidelines
2014. Diabetic Ketoacidosis and Hyperglycemic Hyperosmolar State.
Pediatr Diabetes 2014;15:154-79.
2. Yu H-YE, Agus M, Kellogg MD. Clinical utility of
Abbott Precision Xceed Pro ketone meter in diabetic patients. Pediatr
Diabetes. 2011;12:649-55.
3. Janssen MJ, Hendrickx BH, Habets-van der Poel CD,
van den Bergh JP, Haagen AA, Bakker JA. Accuracy of the Precision?
point-of-care ketone test examined by liquid chromatography tandem-mass
spectrometry (LC-MS/MS) in the same finger stick sample. Clin Chem Lab
Med. 2010;48:1781-4.
4. b-Hydroxybutyrate
(Ketone Body) Colorimetric Assay Kit Cayman Chemical.
https://www.caymanchem.com/product/700190. Accessed March 24, 2015.
5. Fulop M, Murthy V, Michilli A, Nalamati J, Qian Q,
Saitowitz A. Serum beta-hydroxybutyrate measurement in patients with
uncontrolled diabetes mellitus. Arch Intern Med. 1999;159:381-4.
6. Ham MR, Okada P, White PC. Bedside ketone
determination in diabetic children with hyperglycemia and ketosis in the
acute care setting. Pediatr Diabetes. 2004;5:39-43.
7. Rewers A, McFann K, Chase HP. Bedside monitoring
of blood b-hydroxybutyrate
levels in the management of diabetic ketoacidosis in children. Diabetes
Technol Ther. 2006;8:671-6.
8. Wolfsdorf JI. The International Society of
Pediatric and Adolescent Diabetes guidelines for management of diabetic
ketoacidosis: Do the guidelines need to be modified? Pediatr Diabetes.
2014;15:277-86.
9. Turan S, Omar A, Bereket A. Comparison of
capillary blood ketone measurement by electrochemical method and urinary
ketone in treatment of diabetic ketosis and ketoacidosis in children.
Acta Diabetol. 2008;45:83-5.
|
|
|
 |
|

