|
|
|
Indian Pediatr 2016;53: 149-153 |
 |
Placebo-controlled Randomized Trial Evaluating
Efficacy of Ondansetron in Children with Diarrhea and Vomiting:
Critical Appraisal and Updated Meta-analysis
|
|
Source Citation: Danewa AS, Shah D, Batra P, Bhattacharya SK, Gupta
P. Oral ondansetron in management of dehydrating diarrhea with vomiting
in children aged 3 months to 5 years: A randomized controlled trial. J
Pediatr. 2016; 169:105-9.
Section Editor: Abhijeet Saha
|
|
Summary
In this double blind randomized placebo-controlled
trial from New Delhi, India, 170 children (age 3 mo to 5 y) with acute
diarrhea with vomiting and some dehydration were randomized equally to
receive either single dose of oral ondansetron or placebo in addition to
standard management of dehydration according to World Health
Organization guidelines. Failure of oral rehydration therapy (ORT),
administration of unscheduled intravenous fluids, and amount of oral
rehydration solution intake in 4 hours were the primary outcomes.
Failure of ORT was significantly less in children receiving ondansetron
compared with those receiving placebo (31% vs 62%; P<0.001;
RR 0.50, 95% CI 0.35, 0.72). The oral rehydration solution consumption
was significantly more in the ondansetron group (645 mL vs 554 mL;
mean difference 91 mL; 95% CI: 35, 148 mL). Patients in the ondansetron
group also showed faster rehydration, lesser number of vomiting
episodes, and better caregiver satisfaction. The authors concluded that
a single oral dose of ondansetron, given before starting ORT to children
<5 years of age having acute diarrhea and vomiting, results in better
oral rehydration.
Commentaries
Evidence-based Medicine Viewpoint
Relevance: Clinical experience suggests that
vomiting is often a significant barrier to successful oral rehydration
therapy (ORT) in children with acute gastroenteritis. Vomiting can
result in reluctance among family members/caregivers to administer
adequate quantity of oral fluid; it sometimes impels physicians to
prescribe intravenous fluids to avoid the delays associated with oral
rehydration; and it also creates difficulties for individual children to
accept oral rehydration salt (ORS) solution in appropriate amounts.
Available systematic reviews suggest that anti-emetic therapy
administered in conjunction with ORS solution (ORS) may enhance the
efficacy of ORT [1-4]. Against this background, the recent trial by
Danewa, et al. [5] comparing oral ondansetron versus
placebo for management of dehydration among children with diarrhea
having associated vomiting, is a significant value addition to existing
literature. The authors identified some of the lacunae in existing
knowledge [1] and addressed these. Table I summarizes the
main features of the trial.
Table I Summary of the Trial
|
Objective |
To
compare the efficacy and safety of orally administered
ondansetron versus placebo, for the management of dehydration in
children prescribed oral rehydration therapy for diarrhea and
vomiting. |
| Study
design and setting |
Single center, placebo controlled double-blinded, randomized
controlled trial in a tertiary care, teaching hospital in Delhi,
India. |
|
Population (P) |
Inclusion criteria: Children (3mo-5y) with diarrhea (<14 d
duration) with WHO-defined ‘some dehydration’ and >2 episodes
vomiting in the preceding 6 h prior to presentation. Exclusion
criteria: Children with severe acute malnutrition, altered
sensorium, seizures, peripheral edema, paralytic ileus, previous
receipt of any anti-emetic medication and/or prior intravenous
fluids. |
|
Intervention (I) |
Ondansetron (oral) (0.2 mg/kg) just before starting ORT. Unlike
previous trial, the investigators used precise rather than
empiric dosage. |
|
Comparison (C) |
Placebo (oral) administered in a similar dose. |
|
Outcomes (O) |
Efficacy: Failure of ORT (defined as persistence or worsening of
dehydration after 4 h of therapy); Need for intravenous fluids
(with clear criteria for the same); Total volume of ORS accepted
within 4 hours of treatment; Vomiting episodes; Duration of
dehydration; Parent/caregiver satisfaction with treatment.
Safety: Adverse events (diarrhea, headache, rash) recorded by
investigators during therapy. |
|
Time-frame (T) |
All
outcomes were assessed within a short time-frame of 8 hours.
|
|
Sample size |
Sample size was calculated a priori for each of the three
primary outcomes, and the total number randomized was adequate
to cover for any drop outs following randomization.
|
|
Similarity of groups at |
Children in the intervention and comparison groups had similar
characteristics at baseline in terms of age |
|
baseline |
distribution, gender, duration of diarrhea, dehydration status,
nutritional status, and vomiting frequency. |
Critical appraisal: Critical appraisal of the
trial [5] adapting various standard tools [6,7] is summarized in
Table II. The trial fulfilled all criteria for low risk
of bias. It is interesting to note that children in the ondansetron
group could take 75 mL/kg fluid over 4 hours. Incidentally this is the
exact target volume for children with ‘some dehydration’. In contrast,
those in the placebo group could take an average of 63.7 mL/kg in the
same duration. This means that in real world situations, children having
vomiting are unable to accept the required volume of ORS solution. While
this readily explains why nearly two-thirds of children in the placebo
group required another round of ORT or intravenous fluids, it also
suggests that current protocols recommending 75 mL/kg ORS solution may
be chasing a futile goal in such children. The issue is somewhat
complicated by the fact that majority of participants in this trial were
infants receiving breast milk. Since the number of breastfeeding infants
in each group and estimation of number/volume of feeds was not measured,
its implications are unclear.
Table II: Critical Appraisal of The Trial
| Trial
Parameter |
How it was
Done |
Interpretation |
| Randomization |
The allocation
sequence was generated by a computer program. |
Adequate |
|
Varying block
sizes were used to allocate participants. |
|
| Allocation
concealment |
The allocation
sequence was not revealed to anyone involved in the |
Adequate |
|
study.
Intervention and placebo were made available in identical
bottles |
|
|
labelled with
a code representing the allocation. |
|
| Blinding |
Participants,
their parents, and professionals who delivered the |
Adequate |
|
intervention,
managed the children, and assessed the outcomes, were
|
|
|
all blinded.
The intervention and placebo were prepared to have similar
|
|
|
concentration,
taste, colour, and odour. They were packaged in identical
|
|
|
bottles with no distinguishing features. The same volume (mL/kg)
was |
|
|
administered
to both groups. |
|
| Selective
outcome reporting |
All relevant
short-term outcomes were included in this trial. |
Adequate |
| Incomplete
outcome |
Of the 170
participants randomized, only 3 (1.8%) did not complete the
|
Adequate |
| reporting |
study per
protocol. This low attrition is probably owing to the short term
|
|
|
outcomes in
the trial. |
|
| Statistical
methods |
Appropriate
statistical tests were used for most outcomes. Per protocol |
Adequate
|
|
analysis was
chosen, rather than intention-to-treat analysis. However,
|
|
|
as the
attrition rate was very low, it may not compromise the validity.
|
|
| Main results (Ondansetron
|
Failure of
ORT: RR 0.50 [95% CI 0.35, 0.72], NNT rounded to 4 Need |
Ondansetron
|
| vs placebo) |
for
intravenous. fluids: RR 0.56 [95% CI 0.30, 1.07], NNT 9Volume of
|
superior for
all |
|
ORS accepted:
Mean difference 91 mL [95% CI 35 mL, 147 mL] |
outcomes
except |
|
Vomiting
episodes: Mean difference -1.80 [-2.5, -1.1] Duration of
|
need for |
|
dehydration:
These are presented as survival curves and demonstrate |
intravenous
fluids. |
|
superiority of
ondansetron starting from 3 hours after administration.
|
|
|
Parent/care-giver satisfaction: Statistically significant
superiority |
|
|
with
ondansetron for each component. However, overall score not
|
|
|
presented;
hence need not be synonymous with clinical significance.
|
|
|
Adverse
events: No events in either group, hence differences (if any)
|
|
|
cannot be
determined. |
|
| Overall
impression |
Validity: RCT
with a low risk of bias.Results: Clinically meaningful
|
|
|
results for
almost all outcomes.Applicability: Applicable in most
|
|
|
health-care
settings. |
|
|
ORT: Oral rehydration therapy; NNT: Number needed to treat;
RCT: Randomized controlled trial. |
On the other hand, if children were able to take 75
mL/kg ORS solution within 4 hours, why did some require intravenous
fluids? This issue gains even more importance considering that all the
previous trials and systematic reviews on this subject reported
statistically significant reduction in the need for intravenous
rehydration. The absence of this finding here [5] necessitates updating
current systematic reviews.
Literature search was conducted through PubMed and
the Cochrane Library (search terms: ondansetron diarrhea) on 14 th
January 2016, to identify randomized controlled trials (RCTs) comparing
ondansetron (oral) versus placebo in children with diarrhea and
vomiting, for clinically meaningful outcomes. Trials reporting studies
in children with specific causes of diarrhea (such as irritable bowel
syndrome) were excluded. A total of 141 and 104 citations, respectively
were found. Screening by title, abstract and full text resulted in
identifying 5 eligible RCTs, including the current trial [5,8-11]. One
trial [12] was excluded as it used intravenous ondansetron.
Web
Table I summarizes the key features of the additional
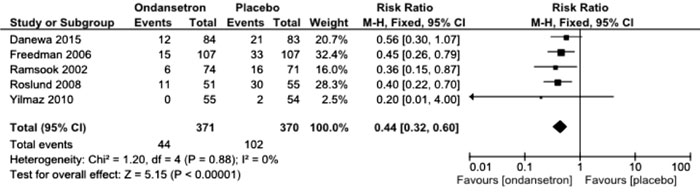
trials. Fig. 1 presents the updated meta-analysis
incorporating data from the present trial for the pertinent outcome. The
updated relative risk is 0.44 [95% CI 0.32, 0.60; 5 trials, 741
participants; I2=0),
confirming that ondansetron reduces need for intravenous fluids by about
55%.
 |
|
Fig. 1 Updated meta-analysis of
ondansetron versus placebo for need of intravenous fluids.
|
Although ondansetron is perceived as a relatively
safe medication, it has the potential to create unpleasant [8,9,13] and
even dangerous side effects [14-16]; although the dose, route, and
participant characteristics may have a bearing. Rarer side effects may
not be observed in a trial with limited participants; this point has
been emphasized by the investigators also [5]. In the absence of a
surveillance system to identify adverse events, physicians themselves
should carefully monitor and report adverse effects. Since orally
administered ondansetron has a short half-life of 3-4 hours [17], it may
be prudent to monitor recipients for at least 24 hours. It is pertinent
that two professional European societies have recommended caution before
ondansetron is routinely used [18].
Extendibility: This well-designed and
well-executed RCT was conducted in a setting familiar to most healthcare
facilities in India and much of the developing world. Participant
selection, intervention adminis-tration, healthcare setting, and outcome
monitoring were similar to the real-world scenarios in centers equipped
to handle acute diarrhea and dehydration. Therefore, the results are
readily extendible to similar settings across the world. It may be
possible to cautiously extend the results to field/community settings
where a combination of ondansetron and ORT administration may begin at
home/primary centres before/while the dehydrated child is transferred to
a facility with resources/personnel to administer intravenous fluids if
required. The enthusiasm for using ondansetron should be tempered with
caution concerning its potential adverse effects.
Conclusion: Ondansetron administered before ORT
in children with diarrhea having additional vomiting results in better
rehydration. However, physicians should be careful to monitor and
report (note emphasis) any side effects of ondansetron occurring in
the first 24 hours. These results are not extendible to children
presenting with severe dehydration.
References
1. Fedorowicz Z, Jagannath VA, Carter B.
Anti-emetics for reducing vomiting related to acute gastroenteritis
in children and adolescents. Cochrane Database Syst Rev
2011;9:CD005506.
2. Carter B, Fedorowicz Z. Antiemetic treatment
for acute gastroenteritis in children: An updated Cochrane
systematic review with meta-analysis and mixed treatment comparison
in a Bayesian framework. BMJ Open. 2012;19:2.
3. Freedman SB, Pasichnyk D, Black KJ,
Fitzpatrick E, Gouin S, Milne A, et al. Gastroenteritis
therapies in developed countries: Systematic review and
meta-analysis. PLoS One. 2015;10:e0128754.
4. Freedman SB, Ali S, Oleszczuk M, Gouin S,
Hartling L. Treatment of acute gastroenteritis in children: An
overview of systematic reviews of interventions commonly used in
developed countries. Evid Based Child Health. 2013;8: 1123-37.
5. Danewa AS, Shah D, Batra P, Bhattacharya SK,
Gupta P. Oral ondansetron in management of dehydrating diarrhea with
vomiting in children aged 3 months to 5 years: A randomized
controlled trial. J Pediatr. 2015; Dec 1. pii:
S0022-3476(15)01165-8.
6. Centre for Evidence-Based Medicine. Critical
Appraisal Tools. Available: from: http://www.cebm.net/critical-appraisal/. Accessed January 14,
2016.
7. Cochrane Risk of Bias Tool. Available from: http://ohg.cochrane.org/sites/ohg.cochrane.org/files/uploads/Risk%20of%20bias%20assessment%20tool.pdf.
Accessed January 14, 2016.
8. Roslund G, Hepps TS, McQuillen KK. The role of
oral ondansetron in children with vomiting as a result of acute
gastritis/gastroenteritis who have failed oral rehydration therapy:
A randomized controlled trial. Ann Emerg Med. 2008;52:22-9.
9. Freedman SB, Adler M, Seshadri R, Powell EC.
Oral ondansetron for gastroenteritis in a pediatric emergency
department. N Engl J Med. 2006;354: 1698-1705.
10. Yilmaz HL, Yildizdas RD, Sertdemir Y.
Clinical trial: oral ondansetron for reducing vomiting secondary to
acute gastroenteritis in children – a double-blind randomized study.
Aliment Pharmacol Ther. 2010;31:82-91.
11. Ramsook C, Sahagun-Carreon I, Kozinetz CA,
Moro-Sutherland D. A randomized clinical trial comparing oral
ondansetron with placebo in children with vomiting from acute
gastroenteritis. Ann Emerg Med. 2002;39:397-403.
12. Rerksuppaphol S, Rerksuppaphol L. Efficacy of
intravenous ondansetron to prevent vomiting episodes in acute
gastroenteritis: A randomized, double blind, and controlled trial.
Pediatr Rep. 2010;2:e17.
13. Ondansetron side effects. Available from: http://www.drugs.com/sfx/ondansetron-side-effects.html. Accessed
January14, 2016.
14. Freedman SB, Uleryk E, Rumantir M,
Finkelstein Y. Ondansetron and the risk of cardiac arrhythmias: A
systematic review and post marketing analysis. Ann Emerg Med.
2014;64:19-25.
15. Moazzam MS, Nasreen F, Bano S, Amir SH.
Symptomatic sinus bradycardia: A rare adverse effect of intravenous
ondansetron. Saudi J Anaesth. 2011;5:96-7.
16. Moffett PM, Cartwright L, Grossart EA,
O’Keefe D, Kang CS. Intravenous ondansetron and the QT interval in
adult emergency department patients: An observational study. Acad
Emerg Med. 2016;23:102-5.
17. The Comprehensive Resource for Physicians,
Drug and Illness Information. Available from: http://www.rxmed.
com/b.main/b2.pharmaceutical/b2.1.monographs/CPS-
%20Monographs/CPS-%20(General%20Monographs-%20Z)/ZOFRAN.html.
Accessed January 14, 2016.
18. Guarino A, Ashkenazi S, Gendrel D, Lo Vecchio
A, Shamir R, Szajewska H; European Society for Pediatric
Gastroenterology, Hepatology, and Nutrition; European Society for
Pediatric Infectious Diseases. European Society for Pediatric
Gastroenterology, Hepatology, and Nutrition/European Society for
Pediatric Infectious Diseases evidence-based guidelines for the
management of acute gastroenteritis in children in Europe: Update
2014. J Pediatr Gastroenterol Nutr. 2014;59:132-52.
Joseph L Mathew
Department of Pediatrics, PGIMER, Chandigarh, India.
Email: [email protected]
Pediatric Gastroenterologist’s Viewpoint
Vomiting associated with acute gastroenteritis is a
distressing symptom for children and their parents. Persistent vomiting
is also one of the main causes of failure of oral rehydration therapy
and need for intravenous rehydration. Decision of using antiemetic drugs
should be guided by their efficacy, side effects and cost. A cochrane
review published in 2011 and a systemic review published in 2012
concluded that use of ondansetron when compared to placebo increased the
proportion of patients with cessation of vomiting (RR 1.44, 95% CI 1.29,
1.61), reduced the need of immediate hospitalization (RR 0.40, 95% CI
0.19, 0.83) and need for intravenous rehydration (RR 0.41, 95% CI 0.29,
0.59) [1,2]. All studies done so far were done in emergency department,
in children with persistent vomiting and mild to moderate dehydration.
There is lack of evidence from ambulatory settings, in children with
vomiting and no dehydration, and in children with moderate to severe
acute malnutrition. Most of studies have used a single dose. Studies
have also reported prolongation of diarrhea in children who received
ondansetron [1]. There is also some concern regarding prolongation of QT
interval in patients with potential electrolyte abnormalities who
receive intravenous ondansetron [3].
In the present study, authors evaluated the role of a
single dose of oral ondansetron in facilitating successful rehydration
of under-five children. This study also reported similar efficacy by
demonstrating lesser failure of ORT (31% vs 62%) and lesser need
of intravenous fluids. This study is most probably the first
double–blind randomized placebo controlled trial on this topic from a
developing country. Present study confirms efficacy of single oral dose
of ondansetron on cessation of vomiting, resulting in better oral
rehydration and parents’ satisfaction. At present there is lack of
evidence for repeated doses, use in ambulatory settings, and in children
with malnutrition.
References
1. Fedorowicz Z, Jagannath VA, Carter B.
Anti-emetics for reducing vomiting related to acute gastroenteritis
in children and adolescents. Cochrane Database Syst Rev.
2011;9:CD005506.
2. Carter B, Fedorowicz Z. Anti-emetic treatment
of acute gastroenteritis in children: An updated Cochrane systematic
review with meta-analysis and mixed treatment comparison in a
Bayesian framework. BMJ Open. 2012;2:e000622.
3. FDA. FDA Drug Safety Communication: Abnormal
heart rhythms may be associated with use of Zofran (ondansetron).
Available from: http://www.fda.gov/Drugs/DrugSafety/ucm271913.htm. Accessed
January 10, 2015.
Praveen Kumar
Department of Pediatrics , LHMC, New Delhi, India.
Email:
[email protected]
Pediatrician’s Viewpoint
Oral rehydration therapy (ORT) has been the
cornerstone of all the diarrhea treatment protocols since 1970s.
However, in this era of indiscriminate use of antibiotics and other
drugs, ORT is being grossly underused. One of the major barriers to ORT
is vomiting which makes pediatricians prefer intravenous fluids many a
times only for parental reassurance. Literature suggests that wealthier
family children are 1.5 times less likely to receive oral rehydration
salt (ORS) solution. Till ORS is made more palatable, we have to rely on
other cost-effective strategies to promote its use.
In this study, the authors have carried out a
systematic randomized controlled trial (RCT), and supported the use of
single dose of ondansetron in acute diarrhea with vomiting for
successful delivery of ORT. However, lack of follow-up to see
readmission rates or assessment for worsening of diarrhea, as reported
in previous studies, has not been done.
In the Indian context, it could be an excellent step
to scale up ORS use as motivating parents and even healthcare providers
to give ORT despite vomiting is not easy, and traditional antiemetics
have been marred with side effects. However, we should resist from a
tendency to jump to this drug as it was originally intended for severe
vomiting in chemotherapy and post-operative patients. More robust
studies are needed to address the concerns of safety and benefit in
ambulatory settings, and in select group of children such as those with
severe malnutrition and other co-morbities.
Shivani Deswal
Department of Pediatrics,
PGIMER, Dr RML Hospital,
New Delhi, India.
Email: [email protected]
|
|
|
 |
|

