|
|
|
Indian Pediatr 2015;52:
152-154 |
 |
Recurrent Kawasaki Disease
|
|
Pramila Verma, Neeti Agarwal and Mahesh Maheshwari
From Department of Pediatrics, People’s College of
Medical Sciences & RC, Bhanpur, Bhopal, India.
Correspondence to: Dr Pramila Verma, Associate
Professor Department of Pediatrics, Sr MIG-B-10, Peoples campus, Bhanpur,
Bhopal India.
Email: [email protected]
Received: July 14, 2014;
Initial review: August 21, 2014;
Accepted: December 06, 2014.
|
|
Background:
Recurrent Kawasaki disease is rare. Case characteristics: An
eight-month old infant had classic Kawasaki disease with transient
coronary artery dilatation. Observations: Recurrence of
incomplete Kawasaki disease after two years of initial diagnosis.
Outcome: The index episode of Kawasaki disease was
resistant to single infusion of immunoglobulin, while repeat episode
responded within 24 hours of institution of therapy. Message:
Early recognition of recurrent Kawasaki disease requires a high index of
suspicion.
Keywords: Incomplete Kawasaki disease, Outcome, Recurrence,
Resistant.
|
|
K
awasaki disease (KD) is an acute, self-limiting,
medium-size vessel vasculitis of unknown etiology that predominantly
involves the skin, mucous membranes, lymph nodes and coronary arteries.
Standard therapy of KD is with a single intravenous infusion of
immunoglobulins (IVIG) and high-dose aspirin until the acute phase
reactants normalize. IVIG-resistant KD, which occurs in approximately
15% of children, can be defined as the persistence of fever beyond 36
hours of the initial IVIG infusion, and mandates a 2nd or even 3rd dose
of IVIG [1-3]. Recurrent KD is mostly reported in Japan and the US,
occurring in 3-4% and 0.8% of cases, respectively [4], but is only
rarely reported from India [5].
Case Report
An 8-month-old boy was referred to us with cough and
cold for 15 days, fever for 5 days, and loose motions for 2 days. On
examination, he was irritable and febrile. He had tachypnea and
tachycardia with normal blood pressure. His eyes and oral mucosa were
injected, and he had strawberry tongue. His hands and feet were
edematous. Systemic examination was unremarkable. The BCG scar mark was
inflamed.
Hematological investigations revealed hemoglobin (Hb)
7.5 g/dL, total leucocyte count (TLC) 36.8×10 9/L,
P:76%, L:22%, platelet count 400x109/L,
C-reactive protein (CRP) 12 mg/dL, erythrocyte sedimentation rate (ESR)
38 mm/h, aspartate aminotransferase (AST) 15.6 U/L, alanine
aminotransferase (ALT) 10.0 U/L, serum sodium 137 meq/L, potassium 3.6
meq/L, serum albumin 2.3 g/dL, blood urea 25.2 mg/dL, and serum
creatinine 0.72 mg/dL. Routine microscopy of urine and cerebrospinal
fluid was normal. Antistreptolysin O (ASO) titer and Mantoux test were
negative. Malarial parasites were not seen in smear. Chest X-ray
showed cardiomegaly. Abdominal ultrasound was normal. He received
injections of ceftriaxone, amikacin, metronidazole and gatifloxacin for
four days before being referred to us. In our hospital, injections of
meropenem, vancomycin and artesunate were administered empirically.
Inspite of 10 days therapy, fever was unresponsive, and the child was
provisionally diagnosed as KD. Two-dimensional echocardiography
(2D-Echo) revealed left coronary artery dilatation (6.9 mm), no fistula,
and mild pericardial effusion with ejection fraction of 60%.
Subsequently, IVIG (2 g/Kg stat dose) and aspirin (85 mg/kg/day in
divided doses) were administered. Fever was persistent even after 36
hours of IVIG, in view of resistant KD; a second dose of IVIG was given
[2]. Consequently fever subsided within 24 hours of the second dose of
IVIG. Aspirin was prescribed at high doses till acute phase reactants
normalized (7 weeks), following which the dose was tapered to 5
mg/kg/day. Echocardiography at 4 months revealed reduction in left
coronary artery dilation (3.4 mm) that completely resolved at 9 months;
aspirin was stopped subsequently.
The child again presented at three years of age
(after 2 years of first episode) with fever for four days, pain in legs
and swelling of hands and feet for two days. He had no history of cold,
cough, allergy, insect bite or drug intake. On examination, the child
was febrile and irritable with tachycardia and normal blood pressure.
His oral mucosa, tongue and pharynx were diffusely injected; ulcers,
enanthem and exudates were not noted. Right sided cervical
lymphadenopathy (>1.5 cm) along with dry, crusted and cracked lips were
present. Edema was noted over hands and feet. Skin examination did not
revealed eruptions or rashes. Rest of the systemic examination was
normal.
Laboratory tests revealed Hb 8.7 g/dL, TLC 16.8 ×10 9/L
with 73% neutrophils, platelet count 521×109/L,
serum albumin 3.2 g/dL, AST 154.0 U/L, ALT 159.7 U/L, ESR 48.0 mm/h, CRP
4.8 mg/dL, Serum sodium 128 meq/L and potassium 3.9 meq/L; ASO titer was
negative. Peripheral smear for malarial parasite and Mantoux test were
negative. Blood culture was sterile. Renal function tests and routine
microscopic examination of urine was normal. Chest X-ray and
abdominal ultrasound was normal. The child was administered intravenous
ceftriaxone, oral antihistaminics and antipyretics. Despite 72 hours of
therapy, fever persisted and there was worsening of oral mucosal
congestion and swelling of extremities. Widal and malaria antigen tests
were negative. Subsequently, child developed periungal desquamation of
fingers, and the child was diagnosed as recurrent KD with incomplete
presentation (>5d fever along with 3 clinical criteria: changes of
mucosae of oropharynx, cervical lymphadenopathy and changes of
peripheral extremities). Concomitant echocardiogram revealed normal
coronary arteries. On the 7th day of fever, the child was administered
with IVIG (2 g/Kg/day) and aspirin (80 mg/Kg/day) in three divided
doses. Defervescence was achieved within 24 hours and remaining features
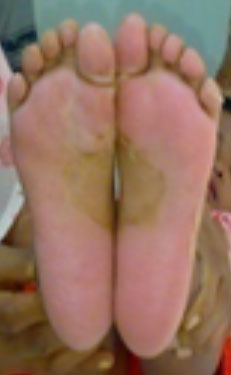
improved over next 2 days. Follow-up after 15 days revealed desquamation
of skin of both soles (Fig. 1). Skin peeling was in sheets
and vastly intense to involve the entire sole, which further
substantiated our diagnosis. Coronary arterial abnormalities (CAA) were
not noted in 2D-echo at 6 weeks, 6 months and 12 months follow-up. The
child is being followed up regularly for the last four years without any
symptoms.
 |
|
Fig. 1 Desquamation of skin over the
soles during second episode of Kawasaki disease. (Color image at
website)
|
Discussion
KD presents with classic or incomplete manifestations
and is diagnosed after excluding similar disease conditions with the
help of clinical criteria proposed by the American Heart Association
[3]. The differential diagnosis of KD includes viral infections
(measles, adenovirus, rubella, erythema infectiosum, infectious
mononucleosis and herpangina) which shares acute oro-pharyngitis, fever
and cervical adenopathy but with less evidence of systemic inflammation
and lack the extremity changes as seen in KD. Systemic onset juvenile
idiopathic arthritis mimics KD but lack of arthritis in the child over
long term follow up excluded it. Absence of skin rashes negated scarlet
fever, rickettsial infections and polyarteritis nodosa. The blood
pressure was normal, ruling out streptococcal toxic-shock syndrome.
Thus, we diagnosed the case as recurrent KD with incomplete
presentation, which was supported by laboratory reports. Sheetlike skin
peeling is pathognomonic of KD and additionally supported our diagnosis
[2]. Both the episodes responded well to IVIG. However, the initial
episode was resistant to the single infusion of IVIG and responded only
after repeat dose [1]. If the symptoms of KD persist or recur after IVIG
therapy, macrophage activation syndrome as a complication of KD should
be considered. However it was excluded, as hepatosplenomegaly and
pancytopenia were not noted in the child during entire course of
illness.
The risk factors predictive of recurrent KD in a
child are: younger age (£2
years) at the onset, male sex, treatment with IVIG [6,7], longer
durations of fever, lower hemoglobin levels [8] and presence of CAA at
the first episode [4,9]. All these risk factors for recurrence at the
first episode were present in our case as well. High levels of
transaminitis at first episode also poses risk for recurrent KD but were
normal in our patient.
Hirata, et al. [8] reported recurrence of KD
within a year while, the present case had recurrence after two years of
first episode. Recurrence of KD and Incomplete KD are generally
associated with CAA [2,3]; however, this was not seen in our case. Kato,
et al. [2] reported CAA occurrence in 20% children of untreated
KD in Japan. Daniels, et al. [10] reported that undiagnosed or
partially treated KD in childhood contributes CAA in 5% of adults in the
USA.
Kawasaki disease should be included in the
differential diagnosis of fever unresponsive to appropriate therapy, as
all the criteria may not appear simultaneously but emerge serially, more
so in a child with previous history of Kawasaki disease.
Contributors: PV: Literature search and review,
manuscript review, manuscript editing, drafting the article and patient
management. She will act as guarantor; NA: Editing the manuscript,
managed the patient and revising the article critically for important
intellectual content; MM: Acquisition and interpretation of data,
managed the patient and edited the manuscript. All authors have approved
the final manuscript.
Funding: None; Competing interests: None
stated.
Reference
1. Singh S, Kawasaki T. Kawasaki disease - An Indian
perspective. Indian Pediatr. 2009;46:563-71.
2. Son MB, Newburger JW. Kawasaki Disease. In:
Kliegman RM, Stanton BF, Geme JW, Schor NF editors. Nelson Textbook of
Pediatrics. 19th ed. Philadelphia: Elsevier; 2011. p. 862-7.
3. Newburger JW, Takahashi M, Gerber MA, Gewitz MH,
Tani LY, Burns JC, et al. American Heart Association Scientific
Statement Diagnosis, treatment, and long-term management of Kawasaki
disease: Circulation. 2004;110:2747-71.
4. Yang HM, Du ZD, Fu PP. Clinical features of
recurrent Kawasaki disease and its risk factors. Eur J Pediatr. 2013;172:1641-7.
5. Balasubramanian S, Ganesh R. Recurrent kawasaki
disease. Indian J Pediatr. 2009;76:848-9.
6. Yanagawa H, Nakamura Y, Yashiro M, Ojima T,
Tanihara S, Oki I, et al. Results of the nationwide epidemiologic
survey of Kawasaki disease in 1995 and 1996 in Japan. Pediatrics.
1998;102:65.
7. Nakamura Y, Hirose K, Yanagawa H, Kato H, Kawasaki
T. Incidence rate of recurrent Kawasaki disease in Japan. Acta Paediatr. 1994;83:1061-4.
8. Hirata S, Nakamura Y, Yanagawa H. Incidence rate
of recurrent Kawasaki disease and related risk factors: From the results
of nationwide surveys of Kawasaki Disease in Japan. Acta Paediatr.
2001;90:40-4.
9. Chen CY, Wu JR. Relapse of Kawasaki disease: A
case report. Gaoxiong Yi, Xue Ke, Xue Za Zhi. 1989;5:189-93.
10. Daniels LB, Tjajadi MS, Walford
HH, Jimenez-Fernandez S, Trofimenko V, Fick DB, et al. Prevalence
of Kawasaki disease in young adults with suspected myocardial ischemia:
Clinical perspective. Circulation. 2012;125:2447-53.
|
|
|
 |
|

