|
|
|
Indian Pediatr 2015;52: 119 -124 |
 |
Predictors of Survival in Children With
Methymalonic Acidemia With Homocystinuria in Beijing, China:
A Prospective Cohort Study
|
|
Li Qiliang, Song Wenqi, *Wang Quan,
#Yang Xinying,
#Li Jiuwei,
‡Sun Qiang,
$Peng Xiaoxia and Wang Peichang
From the Departments of Medical Laboratory,
*Intensive Care Unit, #Neurology, ‡Nephrology and
$Epidemiology
and Biostatistics; Xuanwu Hospital, Beijing Children’s Hospital, Capital
Medical University, China.
Correspondence to: Dr Wang Peichang, Department of
Medical Laboratory, Xuanwu Hospital, Capital Medical University,
Beijing, 100053, China.
Email: peichangwang@yahoo.com
Received: February 28, 2014;
Initial Review: June 13, 2014;
Accepted: November 07,2014.
|
Objective: (i) To determine whether clinical features and
biochemical parameters help to predict survival of methylmalonic
acidemia with homocystinuria; (ii) To find the cutoff values of
biochemical parameters for predicting survival of methylmalonic acidemia
with homocystinuria.
Design: A prospective cohort study.
Setting: A pediatric tertiary hospital in
Beijing; all patients were followed until death or June 2013.
Subjects: 45 pediatric patients diagnosed with
methylmalonic acidemia with homocystinuria between 2006 and 2012.
Outcome measures: The data of clinical
characteristics and pretreatment biochemical parameters were collected.
The Cox regression analysis was performed to identify independent risk
factors for survival of patients with methylmalonic acidemia and
homocystinuria. The best cutoff values for these independent factors
were determined by the receiver characteristic curve.
Results: Newborn onset (OR=6.856,
95%CI=2.241-20.976, P=0.001), high level of methylmalonic acid in
urine (OR=1.022, 95%CI=1.011-1.033, P<0.001), and high level of
urea in serum (OR=1.083, 95%CI=1.027-1.141, P=0.003) were
independent negative risk factors for survival of patients with
methylmalonic acidemia and homocystinuria. The cutoff values of maximum
predictive accuracy of methylmalonic acid in urine and urea in serum
were respectively 5.41 mmol/mmol creatinine and 7.80 mmol/L by receiver
operating characteristic curve analysis.
Conclusion: The patients of methylmalonic
acidemia with homocystinuria tend to have an adverse outcome if they
have newborn onsets. Elevated urea and urinary methylmalonic acid are
predictors of adverse outcomes for the patients. They show similar
effect for predicting severe adverse prognosis. The combination of
methylmalonic acid in urine concentration and urea in serum
concentration provided the most accurate predictive tool.
Key words: Hemocysteine, Methylmalonic acid, Outcome.
|
|
M
ethylmalonic acidemia (MMA) is a rare autosomal
recessive metabolic disease due to a defect of the mitochondrial enzyme
methylmalonyl-CoA mutase (MCM) which converts methylmalonyl-coenzyme A (CoA)
into succinyl-CoA, or a defect in the metabolism of 5’-deoxyadenosylcobalamin,
the cofactor of MCM [1,2]. The prevalence of MMA is 1/29,000 in the
United States and 1/61,000 in Canada, but still unknown in China [3],
though estimated incidence in mainland China is 1/26,000 [1]. According
to serum total homocysteine, MMA is divided into isolated MMA and MMA
with homocystinuria. MMA with homocystinuria has been reported to common
in China [4]. The prognosis of MMA with homocystinuria is poor [5]. We
planned this prospective cohort study to validate the performance of
clinical characteristics and biochemical parameters to predict survival
in chinese children with MMA homocystinuria.
Methods
This prospective cohort study was conducted from July
2006 to February 2012 at a pediatric tertiary-care hospital in Beijing.
Study protocol was approved by Ethics Committee of the hospital and
written informed consent was obtained from parents. Urinary organic
acids profiles were analyzed by the Gas chromatography–mass spectrometry
(GC/MS) in all patients who had the signs/symptoms such as epilepsy,
recurrent vomiting, impaired consciousness, recurrent difficulty with
feeding, both lower extremities edema, mental retardation or regression.
If the level of urinary methylmalonic acid was continuously higher than
100 folds of the normal level (<0.001mmol/mmol creatinine) and a
secondary MMA with vitamin B 12
deficiency was excluded and the serum homocysteine level was higher than
15 mol/L (normal 4-12 mol/L), MMA with homocystinuria was diagnosed
[3,6]. All children diagnosed with MMA with homocystinuria were eligible
for enrolment in the study (Fig. 1).
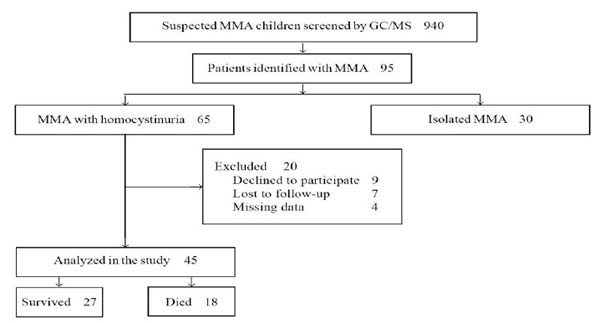 |
|
Fig. 1 Study flow diagram.
|
Details of history and clinical characteristics were
recorded. Pre-treatment biochemical parameters including urinary
methylmalonic acid, serum homo-cysteine, pH, serum creatinine, blood
urea nitrogen, uric acid, alanine aminotransferase, aspartate amino-transferase,
creatine kinase-MB, lactate dehydrogenase,
a-hydroxybutyrate
dehydrogenase, white blood cell, red blood cell, hemoglobin, occult
blood reaction and specific gravity of urine were analyzed. For quality
assurance, three levels of internal controls were run with each batch of
twenty samples. The intra-assay and inter-assay variation were less than
3% and 5%, respectively. The patients received a homogeneous and
standardized medical management [3,4]; intramuscular injection of
vitamin B12 0.5-1 mg/d once
or twice weekly (or oral mecobalamin tablets 1mg/d), folic acid 2.5-5
mg/d, betaine 500-2000 mg/d, and L-carnitine 250-1000 mg/d. All patients
were followed until death or till June 2013 (censor date).
Statistical analysis: All statistical analyses
were performed using SPSS version 13.0. Statistical significance was set
at P<0.05. Categorical variables were compared using the
Chi-square test or Fisher exact test, as appropriate. Continuous
variables were compared using the Student t test if normally
distributed or non-parametric test if non-normally distributed.
Kaplan–Meier survival plots were used to display hypothesized
relationships and data were compared with the log-rank test. The Cox
regression analysis was performed to identify independent risk factors
for survival of MMA with homocystinuria. OR and 95% CI were calculated
for risk estimates. For chemical parameters showing a significant
association with survival, Receiver operating characteristic (ROC) curve
analysis was performed to identify the optimal cutoff points. Areas
under the ROC curve, sensitivity, specificity, positive predictive
value, and negative predictive value were calculated.
Results
During the study period, a total of 65 patients
diagnosed with MMA and homocystinuria were eligible for enrolment. Among
them, 20 were excluded for different reasons (Fig. 1)
and 45 patients (28 males) were enrolled in the study (Table I).
TABLE I Clinical Characteristics of Children with Methylmalonic Acidemia and Homocystinuria (N=45)
|
Characteristic |
Non-survivor |
Survivor |
|
(n=18) |
(n=27) |
|
Male |
9 (50) |
19 (70.4) |
|
*Age at diagnosis, mo |
4.3 (0.1-90.2) |
8.5 (0.1-165) |
|
#Neonatal onset |
6 (33.3) |
2 (7.4) |
|
Family history |
3 (11.1) |
1 (3.7) |
|
$vomiting and/or food refusal and/or impaired
consciousness |
15 (83.3) |
10 (37.0) |
|
All values in n (%) except *median (range); #P<0.05;
$Initial clinical presentation
(P<0.005). |
At the end of the study, 27 patients were still
surviving. The cause of death was renal failure in 10 patients, multiple
organ failure in 5 patients, and decompensated metabolic acidosis in 3
patients. Clinical manifestations of 45 patients at diagnosis included
epilepsy (n =17), recurrent vomiting (n =10), impaired
consciousness (n =10), recurrent difficulty with feeding (n =9),
both lower extremities edema (n=7), mental retardation or regression (n
=5), jaundice (n=5), anemia (n =4), precordial discomfort (n
= 3), hypotonia (n =2), tremor (n = 1), dysarthria (n
=1), ataxia (n =1), coma (n=1) and abnormal posture (n
=1). The results are shown in Table I. The occurrence
of vomiting, food refusal and impaired consciousness in non-survivors
were more than survivors (P<0.005).
TABLE II Baseline Laboratory Findings of Non-survivors and Survivors Before Clinical Treatment
|
Parameters |
Unit |
Reference range |
Non-survivors (n=18) |
Survivors (n=27) |
P value |
|
MMA |
mmol/mmol creatinine |
0.000-0.001 |
7.74 (0.11-147.16) |
0.34 (0.10-20.87) |
0.002 |
|
SG |
– |
1.003-1.030 |
1.015 (1.003-1.035) |
1.015 (1.005-1.035) |
NS |
|
pH |
pH units |
7.35-7.45 |
7.32 (7.01-7.42) |
7.36 (7.07-7.49) |
0.01 |
|
Ammonia |
µmol/L |
0-54 |
72 (9-700) |
55 (14-280) |
NS |
|
Lactate |
mmol/L |
0.5-2.2 |
2.15 (0.4-4.2) |
2.5 (0.7-4.8) |
NS |
|
HCY |
mmol/L |
1.9-12.98 |
75.45 (16.20-214.80) |
119.10 (35.60-219.80) |
NS |
|
WBC |
109/L |
4.0-10.0 |
5.65 (1.79-12.60) |
6.40 (4.80-16.10) |
NS |
|
RBC |
1012/L |
3.5-5.5 |
3.18 (1.66-5.43) |
3.31 (2.12-4.56) |
NS |
|
HGB |
g/L |
110.0-160.0 |
102.50 (60.0-174.0) |
108.00 (73.0-137.0) |
NS |
|
BUN |
mmol/L |
1.7-7.1 |
8.64 (2.11-16.64) |
3.21 (1.69-10.20) |
0.001 |
|
CR |
µmol/L |
27.0-130.0 |
77.55 (22.10-421.00) |
43.20 (18.90-83.80) |
0.012 |
|
UA |
µmol/L |
119.0~416.0 |
455 (213.0-1194.8) |
255 (134.0-1113.4) |
0.006 |
|
ALT |
U/L |
5.0-40.0 |
32.35 (12.0-54.0) |
21.00 (8.2-60.6) |
NS |
|
AST |
U/L |
5.0-40.0 |
53.65 (28.40-157.00) |
31.00 (17.50-163.70) |
0.001 |
|
CK-MB |
U/L |
0.0-25.0 |
21.85 (10.0-225.0) |
17.50 (10.0-55.3) |
0.029 |
|
LDH |
U/L |
50.0-240.0 |
314.00 (60.0-1685.0) |
244.00 (134.0-3347.0) |
0.034 |
|
HBDH |
U/L |
80.0-220.0 |
255.00 (89.0-1335.0) |
195.00 (120.0-2886.0) |
NS |
|
Values in median (range) NS – not significant; MMA –
Methylmalonic acid; SG – specific gravity of urine; pH –
arterial blood pH; HCY – Homocysteine; WBC – White blood cells;
RBC – Red blood cells; HGB – Hemoglobin; BUN – Blood urea
nitrogen; CR – Creatinine; UA – Uric acid ; ALT – Alanine
aminotransferase; AST – Aspartate aminotransferase; CK-MB –
Creatine kinase-MB; LDH – Lactate dehydrogenase; HBDH – a-hydroxybutyrate
dehydrogenase. |
Laboratory findings of survivors and non-survivors
are compared in Table II. The primary biochemical
parameters (e.g. urinary methylmalonic acid, pH) were
significantly different between survivors and non-survivors. The
parameters reflecting kidney (e.g. urea, creatinine and uric
acid) and myocardial (e.g. aspartate aminotransferase, creatine
kinase-MB, lactate dehydrogenase) injury were significantly higher in
non-survivors than survivors. In addition, the positive rate of urine
occult blood reaction in non-survivors (12/18) was significantly higher
than survivors (6/27) (P<0.01).
TABLE III Prognostic Factors Associated With Death in Children with
Methylmalonic Acidemia with Homocystinuria.
|
Variables |
P value |
OR (95%CI) |
|
With newborn onset |
0.001 |
6.856 (2.241, 20.976) |
|
MMA in urine |
0.000 |
1.022 (1.011, 1.033) |
|
BUN in serum |
0.003 |
1.083 (1.027, 1.141) |
|
CI– confidence interval; OR–odd ratio; MMA–Methylmalonic
acid; BUN–Blood urea nitrogen; significance level of 0.05 or
less. |
The multivariate Cox-proportional hazard model was
used to determine independent risk factors of death of patients.
Potential risk factors were preliminary screened by Kaplan–Meier
survival method and clinical knowledge. The alternative risk factors in
the Cox proportional hazard model included with or without newborn
onset, with or without the occurrence of vomiting or food refusal or
impaired consciousness, concentrations of methylmalonic acid in the
urine, the levels of blood urea nitrogen, creatinine, uric acid,
aspartate aminotransferase, and creatine kinase-MB. With newborn onset,
high levels of methylmalonic acid and urea were independent negative
risk factors for survival (Table III). The cut-off values
of maximum predictive accuracy of methylmalonic acid in urine and urea
in serum, respectively were 5.41 mmol/mmol creatinine and 7.80 mmol/L by
Receiver operating characteristic curve analysis. The performance of
methylmalonic acid in urine as an individual factor for identifying
patients at a high risk of death was similar with urea in serum (Table
IV). The combination of methylmalonic acid in urine and urea in
serum provided the most accurate predictive tool (e.g. increased
sensitivity without decreased specificity).
TABLE IV Prognostic Factors for Poor Outcome in Children With Methylmalonic Acidemia and Homocystinuria
|
Finding |
AUC |
Sensitivity (%) |
Specificity ( %) |
PPV(%) |
NPV(%) |
|
Urinary MMA >5.41 mmol/mmol creatinine |
0.778 ± 0.071 |
72.2 |
81.5 |
81.3 |
82.8 |
|
Urea >7.80 mmol/L |
0.787 ± 0.070 |
55.6 |
89.9 |
76.9 |
75.0 |
|
Urinary MMA >5.41 mmol/mmol creatinine |
|
|
|
|
|
|
or Urea >7.80 mmol/L |
0.901 ± 0.035 |
77.8 |
96.3 |
93.3 |
86.7 |
|
AUC – area under the curve; PPV – positive predictive
value; NPV – negative predictive value; MMA – Methylmalonic
acid. |
Discussion
Some retrospective studies reported that the patients
with methylmalonic acidemia had different long term outcomes [4,7]. In
order to find out predictors for survival of MMA with homocystinuria, we
analyzed clinical features and biochemical parameters of 45 Chinese
pediatric patients and assessed the predictive ability.
Among the clinical manifestations, recurrent
vomiting, food refusal, and impaired consciousness were reported by
Zwickler, et al. [8] as life-threatening alarming symptoms of
patients with MMA. Although we also found the more occurrences of
recurrent vomiting, food refusal, and impaired consciousness in
non-survivors, the three symptoms were not individual risk factors for
death of the patients. The possible cause of inconsistency in the two
studies is probable due to different populations. Zwickler, et al.
[8] selected patients diagnosed with isolated MMA as research subjects.
However, our research subjects were children diagnosed as MMA with
homocystinuria. Because the pathogenic mechanisms between isolated
methylmalonic academia and methylmalonic academia with homocystinuria
are different [4,9], the alarming symptoms may have different
predictable values.
Newborn onset was found to be a major clinical
feature to predict negative outcome of MMA with homocystinuria, possibly
because these patients have more severe complications e.g.
decompensated metabolic acidosis, renal failure and multiple organ
failure, or the diagnosis is often missed as clinical symptoms during
neonatal period are not apparent or specific [10]. Thus, irreversible
damage such as organ failure had already occurred by the time these
patients were seen at our center. It is expected that routine newborn
screening can reduce morbidity and mortality by early diagnosis and
early treatment [11,12].
In this study, some biochemical parameters indicated
more metabolic decompensation and more severe renal injury and
myocardial injury in non-survivors than survivors. Hörster, et al.
[15] reported that high concentrations of MMA in urine are a known risk
factor for the development of chronic kidney disease. Moreover,
homocysteine can induce myocardial injury by promotion of endothelial
dysfunction, formation of thromboxane A2, enhancement of platelet
aggregation, reduction in the protective effect of nitric oxide, and the
procoagulant effects [4-17]. The disturbance of the tricarboxylic acid
cycle and respiratory chain may also be involved in the pathogenesis
[18]. In addition, mitochondrial dysfunction, oxidative stress and
disturbances in mitochondrial DNA equilibrium may be associated with
organ injury [19-21].
We found that elevated urinary methylmalonic acid was
one biochemical predictor of adverse outcomes for MMA with
homocystinuria. Ledley, et al. [22] found that children who had
low levels of methylmalonic acid in blood and urine had good outcomes
[22]. However, there is no report about the cut-off value of urinary
methylmalonic acid to predict long term outcomes of MMA with
homocystinuria. In our study, the level of urinary methylmalonic acid
>5.41 mmol/mmol creatinine (5410 folds of normal level) showed high
specificity for identifying patients at increased risk for death.
However, as an independent risk factor, the sensitivity of urinary
methylmalonic acid was limited.
Blood urea nitrogen was the other biochemical
predictor of adverse outcomes for MMA with homocystinuria. Our study
found that renal failure was one of the main causes of the death. Blood
urea nitrogen, an important biochemical parameter reflecting renal
function, showed prediction effect for death of patients. However, it is
very interesting that there is a discrepancy of blood urea nitrogen and
serum creatinine with different prognostic value for outcome. The rise
of blood urea nitrogen had prognostic significance, but serum creatinine
had not. In order to find out whether the rise of blood urea nitrogen
resulted from the prerenal azotemia induced by reduced blood volume, we
used the parameter of specific gravity of urine to evaluate the water
balance in children. The results showed that specific gravity of urine
was not significantly different between the two groups. In addition, the
positive rate of urine occult blood reaction in non-survivors was
significantly higher than survivors. So we think that the rise of blood
urea nitrogen may result from postrenal azotemia. We found that the most
patients were in poor nutrition state because of vomiting and food
refusal, leading to a loss of muscle mass thereby reducing creatinine
synthesis. Therefore, the level of serum creatinine could not reflect
accurately the renal function. The blood urea nitrogen level with the
greatest prognostic ability was 7.80mmol/L, which had a similar
specificity with urinary methylmalonic acid >5.41 mmol/mmol creatinine
for predicting severe adverse prognosis of MMA with homocystinuria. In
addition, combination of elevated urinary methylmalonic acid or elevated
blood urea nitrogen provided better predictive ability by increasing
sensitivity compared with each variable considered separately.
Relevant pathogenic genes (MMACHC, MMADHC, LMBRD1)
were not detected in the present study due to lack of facilities. Thus,
we could not get the information of different gene and different
mutations risk assessment for survival of MMA with homocystinuria.
In conclusion, with newborn onset, higher levels of
urinary methylmalonic acid and blood urea nitrogen increase risk for
death of MMA with homocystinuria. Further studies are needed to validate
performance of pathogenic genes to predict survival of the patients with
MMA and homocystinuria.
Contributors: LQ: participated in the design,
carried out the experimental work, collection and interpretation of the
data and drafted the manuscript; WQ, YX, LJ, SQ: participated in the
design and coordination of experimental work, and acquisition of
clinical data; PX: participated in the study design, data collection,
analysis of data and preparation of the manuscript; WP, SW: carried out
the study design, the analysis and interpretation of data. All
authors approved the manuscript for submission.
Funding: Special Program for Capital Clinical
Research of the Beijing Municipal Commission of Science and Technology,
China (Grant No. Z121107005112008).
Competing interests: None stated.
References
1. Tu WJ. Methylmalonic acidemia in mainland
China. Ann Nutr Metab. 2011;58:281.
2. Carrillo-Carrasco N, Chandler RJ, Venditti CP.
Combined methylmalonic acidemia and homocystinuria, cblC type. I.
Clinical presentations, diagnosis and management. J Inherit Metab
Dis. 2012;35:91-102.
3. Ma X, Zhang Y, Yang Y, Liu X, Yang Z, Bao X,
et al. Epilepsy in children with methylmalonic acidemia:
electroclinical features and prognosis. Brain Dev. 2011;33:790-5.
4. Huang Z, Han LS, Ye J, Qiu WJ, Zhang HW, Gao
XL, et al. Outcomes of patients with combined methylmalonic
acidemia and homocystinuria after treatment. Zhonghua Er Ke Za Zhi.
2013;51:194-8.
5. Rosenblatt DS, Aspler AL, Shevell MI, Pletcher
BA, Fenton WA, Seashore MR. Clinical heterogeneity and prognosis in
combined methylmalonic aciduria and homocystinuria (cblC). J Inherit
Metab Dis. 1997;20: 528-38.
6. Zhang Y, Song JQ, Liu P, Yan R, Dong JH, Yang
YL, et al. Clinical studies on fifty-seven Chinese patients
with combined methylmalonic aciduria and homocysteinemia. Zhonghua
Er Ke Za Zhi. 2007;45:513-7.
7. Cosson MA, Benoist JF, Touati G, Déchaux M,
Royer N, Grandin L, et al. Long-term outcome in methylmalonic
aciduria: a series of 30 French patients. Mol Genet Metab.
2009;97:172-8.
8. Zwickler T, Haege G, Riderer A, Hörster F,
Hoffmann GF, Burgard P, et al. Metabolic decompensation in
methylmalonic aciduria: which biochemical parameters are
discriminative? J Inherit Metab Dis. 2012; 35: 797-806.
9. Vatanavicharn N, Champattanachai V,
Liammongkolkul S, Sawangareetrakul P, Keeratichamroen S, Ketudat
Cairns JR, et al. Clinical and molecular findings in Thai
patients with isolated methylmalonic acidemia. Mol Genet Metab.
2012;106:424-9.
10. Tu W, He J, Dai F, Wang X, Li Y. Impact of
inborn errors of metabolism on admission in a neonatal intensive
care unit – a prospective cohort study. Indian J Pediatr.
2012;79:494-500.
11. Nagaraja D, Mamatha SN, De T, Christopher R.
Screening for inborn errors of metabolism using automated
electrospray tandem mass spectrometry: study in high-risk Indian
population. Clin Biochem. 2010;43:581-8.
12. Sun W, Wang Y, Yang Y, Wang J, Cao Y, Luo F,
et al. The screening of inborn errors of metabolism in sick
Chinese infants by tandem mass spectrometry and gas
chromatography/mass spectrometry. Clin Chim Acta. 2011;412:1270-4.
13. Hörster F, Baumgartner MR, Viardot C,
Suormala T, Burgard P, Fowler B, et al. Long-term outcome in
methylmalonic acidurias is influenced by the underlying defect
(mut0, mut-, cblA, cblB). Pediatr Res. 2007; 62:225-30.
14. Durand P, Lussier-Cacan S, Blache D. Acute
methionine load-induced hyperhomocysteinemia enhances platelet
aggregation, thromboxane biosynthesis, and macrophage-derived tissue
factor activity in rats. FASEB J. 1997;11:1157-68.
15. Woo KS, Sanderson JE, Sun YY, Chook P, Cheung
AS, Chan LT, et al. Hyperhomocyst(e)inemia is a risk factor
for arterial endothelial dysfunction in humans. Circulation.
2000;101:E116.
16. Stamler JS, Osborne JA, Jaraki O, Rabbani LE,
Mullins M, Singel D. Adverse vascular effects of homocysteine are
modulated by endothelium-derived relaxing factor and related oxides
of nitrogen. J Clin Invest. 1993; 91:308-18.
17. Mayer EL, Jacobsen DW, Robinson K.
Homocysteine and coronary atherosclerosis. J Am Coll Cardiol.
1996;27:517-27.
18. Morath MA, Okun JG, Müller IB, Sauer SW,
Hörster F, Hoffmann GF, et al. Neurodegeneration and chronic
renal failure in methylmalonic aciduria—a pathophysiological
approach. J Inherit Metab Dis. 2008; 31:35-43.
19. de Keyzer Y, Valayannopoulos V, Benoist JF,
Batteux F, Lacaille F, Hubert L, et al. Multiple OXPHOS
deficiency in the liver, kidney, heart, and skeletal muscle of
patients with methylmalonic aciduria and propionic aciduria. Pediatr
Res. 2009;66:91-5.
20. Kölker S, Sauer SW, Surtees RA, Leonard JV.
The aetiology of neurological complications of organic
acidaemias—a role for the blood-brain barrier. J Inherit Metab Dis.
2006;29:701-4.
21. Kölker S, Burgard P, Sauer SW, Okun JG.
Current concepts in organic acidurias: understanding intra- and
extracerebral disease manifestation. J Inherit Metab Dis.
2013;36:635-44.
22. Ledley FD, Levy HL, Shih VE, Benjamin R,
Mahoney MJ. Benign methylmalonic aciduria. N Engl J Med.
1984;311:1015-8.
|
|
|
 |
|

