|
|
|
Indian Pediatr 2015;52: 115 -118 |
 |
Effect of Simultaneous
Administration of Oral Polio Vaccine on Local Reaction of BCG
Vaccine in Term Infants
|
|
MMA Faridi and Shitanshu Srivastava
From Department of Pediatrics, Era’s Lucknow Medical
College, Lucknow, UP, India.
Correspondence to: Dr MMA Faridi, E-9 GTB
Hospital, Delhi 110 095, India.
Email: [email protected]
Received: December 30, 2013;
Initial review: February 06, 2014;
Accepted: December 08, 2014.
|
Objective: To study local reaction and to ascertain timing of scar
formation in infants after BCG vaccination at birth, with and without
simultaneous administration of trivalent OPV.
Design: Prospective observational study.
Setting: Teaching hospital in Lucknow, India.
Participants: 152 term neonates born in the
hospital and given BCG and OPV 0-dose simultaneously before discharge,
within 7 days of birth (Group I) , and 122 infants born at home or in
private health facility, not given OPV-0 dose, coming for vaccination
within 7 days of age (Group 2).
Intervention/Observation: Follow up done at 6
week, 10 week, 14 week and 9 months. Local reaction was recorded at the
site of BCG vaccination.
Results: Scar formed in
£14 wks
in 51.3% and 89.3% babies in Group 1 and Group 2, respectively following
BCG vaccination (P<0.001). At 9 months, scar developed in 93.9%
infants in Group I and 94.3% babies in Group II. Abortive reaction and
non-reactors were similar in both groups (P>0.05).
Conclusions: Simultaneous administration of BCG
vaccine with trivalent OPV to term infants in early neonatal period
prolongs the time of scar formation but sequence of local reaction is
not affected.
Keywords: Abortive reaction, BCG, Immunization, Neonate, OPV,
Scar.
|
|
B
CG is routinely administered to all newborn
infants under the Universal Immunization Program. Oral Polio Vaccine
(OPV) is recommended at birth together with BCG vaccine for all
institutional deliveries [1]. Local reaction at the BCG vaccination site
is described as papule, pustule, ulcer, scab, scar and abortive
reaction; it may take 10 weeks to 6 months or more to form a scar [2].
Administering OPV together with BCG might down- regulate the response to
BCG vaccine [3]. The present study was planned to observe local reaction
and time of scar formation in term appropriate-for-gestation age (AGA)
infants, following BCG vaccination within 7 days of birth with or
without simultaneous administration of trivalent OPV.
Methods
The present study was conducted in the Department of
Pediatrics, Era’s Medical College and Hospital, Lucknow, India during
March 2008 to December 2010 after taking approval from the Institutional
Ethics Committee and informed consent from parents. All consecutive term
AGA babies [5] of both sexes born in the hospital with birth weight
2.2-4 kg were recruited. Consecutive neonates who were not delivered in
the study hospital but came for vaccination within 7 days of birth were
screened by history and available investigation reports done in the
antenatal period, and were included if they fulfilled inclusion
criteria. Infants having contact with tuberculosis patient or those born
to mothers suffering from tuberculosis, severe anemia, diabetes,
HIV/AIDS or Hepatitis B and those admitted in the neonatal intensive
care unit for sepsis, intrauterine infection, jaundice, perinatal
asphyxia, congenital malformation or suspected chromosomal anomalies,
and family residing more than 10 km away from the hospital were excluded
from the study.
The institution protocol is to discharge normal
parturient mothers and their babies after 48 hours of birth; in case of
Lower Segment Cesarean Section (LSCS) a mother is discharged on day 5-6
after removing abdominal stitches. BCG vaccine is given only on
Thursdays; rest of the vaccines are administered on all working days.
Therefore, BCG and OPV-0 dose cannot be administered simultaneously to
all infants before discharge from the hospital. In home-delivered
infants, the BCG vaccination is advised within 2 weeks but OPV 0-dose is
not recommended to them under National Immunization Schedule. The study
subjects thus were divided in two groups: Group I: Infants born in the
study hospital and received BCG and OPV 0-dose simultaneously within 7
days of age before discharge; and Group II: Babies born at home or in a
private health facility, who did not receive either OPV-0 dose or Pulse
Polio Program dose, and came to study hospital for immunization within 7
days of age. Only BCG vaccine was given to them.
All mothers were counseled for exclusive
breastfeeding by a nurse or faculty trained in infant and young child
feeding counseling at the time of discharge [6]. They were also
counseled for regular follow-ups and immunization on subsequent visits
as per National Immunization Schedule.
One trained staff nurse with more than 5 years of
experience in the immunization clinic administered 0.1 mL of BCG vaccine
(Danish 1331 strain; BCG laboratory, Guindy Chennai) to all enrolled
babies. The freeze-dried vaccine was reconstituted with normal saline
and given intradermal on the left arm just above the insertion of the
deltoid muscle with a 26-gauge needle and tuberculin syringe to produce
a wheal of 5 mm. The reconstituted vaccine was used within 4 hours and
then discarded. Cold chain was maintained and monitored as per standard
protocol. Oral trivalent polio vaccine Sabin strain (Bharat Biotech) was
administered to babies in Group I simultaneously with BCG. OPV was not
administered to infants belonging to Group II. Cold chain was maintained
and vaccine viability was checked with the help of vaccine vial monitor.
Four follow-ups were done at 6 (+1) week, 10 (+1)
week, 14 (+1 week) and 9 months (+2 weeks). The local reaction at the
vaccination site was recorded as papule, pustule, ulcer, scab, scar, no
reaction, and abortive reaction [2]. Babies who did not have any
reaction at the inoculation site at 14 weeks were labeled as
non-reactor. Infants who did not develop scar by 14 weeks but had
papule, pustule or ulcer were counseled for follow-up at 9 months. Local
reaction was recorded by a single observer in all subjects. If there was
any ambiguity, both authors concurred for abortive reaction or no
reaction.
Statistical analyses: The data were entered in
Microsoft Excel and checked for any inconsistency. Univariate logistic
regression analysis was carried out to find out any relationship of scar
formation at 6, 10, 14 weeks and 9 months with sex and birth weight of
the infants. P-value <0.05 was considered as significant.
Analysis was carried out with SPSS Version 16.0.
Results
Out of 275 babies recruited (Fig. 1),
Group I comprised of 152 consecutive neonates. Four of these infants
were lost to follow-up and 148 infants were included for analysis. Group
II comprised of 123 neonates, who received only BCG. One child was lost
to follow-up due to change of residence as per telephonic conversation.
Hence, data of 122 infants were analyzed.
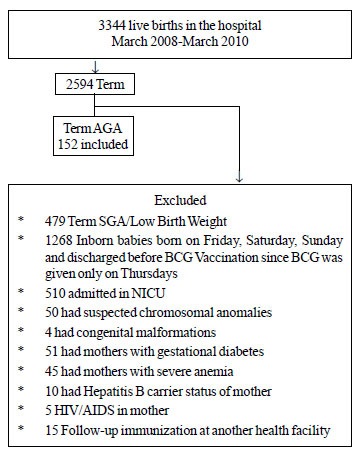 |
|
Fig. 1 Recruitment flow of
participants
|
Number of infants delivered normally, with the help
of forceps and by LSCS, socioeconomic status of the parents and clinical
profile of the mothers such as hemoglobin level, parity and antenatal
check-up status were comparable in both groups. The mean (SD) birth
weight of babies in Group I [2.73(0.30 kg)] and Group II [2.75 (0.32
kg)] was comparable (P=0.57). The mean age of infants at
immunization was 2.6 days (range 1-6 days) and 3.9 days (range 2-7 days)
in Group I and II, respectively. Both groups were sex- and age-matched.
Sex and birth weight of the infants did not affect either the
development of local reaction or time of scar formation in both groups.
The advent and sequence of local reaction was similar
in both groups at 6, 10 and 14 weeks following BCG vaccination (Table
I). A higher (P<0.01) proportion of infants deceloped scar at
6 weeks in Group II infants (25.4%), in comparison to infants of Group I
(3.4%).
TABLE I Local BCG Reaction in Group I (BCG and OPV0 dose) and Group II (only BCG) Infants
|
Local Reaction |
6 wks
Group |
10 wks
Group |
14 wks
Group |
9 months
Group |
|
I No. (%) |
II No. (%) |
I No. (%) |
II No. (%) |
I No. (%) |
II No. (%) |
I No. (%) |
II No. (%) |
|
Induration |
30(20.3) |
5(4.1) |
0 |
0 |
0 |
0 |
0 |
0 |
|
Papule |
54(36.5) |
13(10.7) |
14(9.5) |
5(4.1) |
0 |
0 |
0 |
0 |
|
Pustule |
39(26.4) |
28(22.9) |
43(29.1) |
3(2.5) |
10(6.8) |
1 (0.8) |
0 |
0 |
|
Ulcer |
10(6.8) |
33(27.0) |
30(20.3) |
18(14.8) |
20(13.5) |
4 (3.3) |
0 |
0 |
|
Scab |
0(0.0) |
7(5.7) |
35(23.6) |
2(1.6) |
33(22.3) |
1 (0.8) |
0 |
0 |
|
No reaction |
10(6.8) |
5(4.1) |
8(5.4) |
4(3.3) |
5(3.4) |
4 (3.3) |
5 (3.4) |
4 (3.3) |
|
Abortive reaction |
|
|
|
|
4(2.7) |
3 (2.5) |
4 (2.7) |
3 (2.5) |
|
N= 148 in Group I and 122 in Group II. |
Table II depicts the comparison of scar
formation between infants of Group I and II. The development of scar was
significantly lower among Group I as compared to Group II infants till
14 weeks post-vaccination. The cutaneous reaction at the site of BCG
immunization in the form of either scar or abortive reaction was similar
(96.6% vs 96.8%) in both groups.
TABLE II Comparison of Scar Formation in Two Groups
|
Reaction |
6 wks
Group |
10 wks
Group |
14 wks
Group |
9 months
Group |
|
I No. (%) |
II No. (%) |
I No. (%) |
II No. (%) |
I No. (%) |
II No. (%) |
I No. (%) |
II No. (%) |
|
Scar |
5(3.4) |
31(25.4) |
18(12.1) |
90(73.7) |
76(51.3) |
109(89.3) |
139(93.9) |
115(94.3) |
|
No scar |
143(96.6) |
91(74.6) |
130(87.8) |
32(26.2) |
72(48.6) |
13(10.7) |
9(6.1) |
7(5.7) |
|
OR (95% CI), |
0.10 (0.04-0.27) |
0.05 (0.03-0.09) |
0.13 (0.07-0.24) |
0.94 (0.34-2.60) |
|
P value |
<0.001 |
<0.001 |
<0.001 |
0.91 |
|
*Derived by univariate logistic regressionN = 148 in Group I
and 122 in Group II. |
Discussion
Our study demonstrated that simultaneous
administration of OPV with BCG vaccine at birth may affect local BCG
reaction. The sequence of cutaneous reaction, incidence of abortive
reaction and number of non-reactors were similar in infants given BCG
and OPV together and those who received BCG vaccine alone. However, scar
formation lagged behind at 6, 10, and 14 weeks post-immunization in
infants who received both BCG and OPV together. Eventually scar formed
by 9 months in all infants at the site of BCG inoculation.
Interaction between the two live vaccines, BCG and
OPV, administered simultaneously at birth, was studied by the
development of local cutaneous reaction but effect on in vivo
immune response was not studied by us. The other limitations were
non-randomized design of study, and no blinding of investigators.
OPV is widely administered along with BCG at birth in
developing countries but little is known regarding the adverse impact on
cellular immune responses following vaccination [4]. The development of
scar at the site of BCG vaccination is regarded as an evidence of
successful vaccination [3,7-8] and may be taken as a surrogate marker
for development of immunogenicity [9,10]. It has been reported that the
scar forms in 65.3% to 100% infants following BCG vaccination (7-14). In
all these studies, OPV was not administered simultaneously with BCG
vaccine. Kaur, et al [3]. opined that BCG and OPV given together
at birth might delay scar formation by 3 months of age [3]. Sartono,
et al. [4] demonstrated that mucosal administration of OPV
simultaneously with intradermal BCG vaccination at birth may have
profound immunological consequences. They reported that this may down-
regulate response to BCG in the form of fewer numbers of scar formation,
reduced scar size, reduced in vivo response to purified protein
derivative (PPD) and significantly lower IL-13 and IFN- g
and a tendency to have lower IL-10 in response to PPD at 6 weeks
following immunization [4]. Jensen, et al. [15] in a recent study
have also shown that giving OPV and BCG together to neonates led to
significantly lower prevalence of IFN-g
and reduced levels of interleukin-5 to PPD, implying that OPV affected
both Th-1 and Th-2 cytokine responses. Most BCG efficacy studies were
done prior to the introduction of OPV at birth. It may be speculated
that the addition of OPV to BCG at birth has further compromised the
protection induced by BCG [4]. After BCG vaccination, a cascade of
reaction takes place where IL-2, interferon-g
and TNF-a are
predominantly secreted. Inflammation and suppuration at the site of BCG
vaccination are mediated through interleukins [16]. Which of these
reaction/s or cascade is affected by OPV, if administered
simultaneously, at the time of BCG vaccination is an area of research.
We conclude that simultaneous administration of OPV
with BCG vaccine delays scar formation, but it does not affect
sequential development of the local reaction or proportion of
non-reactors.
Contributors: Both authors participated in study
design, data collection and analysis, and manuscript writing.
Funding; None; Competing interests: None
stated.
|
What Is Already Known?
•
The formation of scar at the site of BCG vaccination is
regarded as an evidence of successful immunization.
What This Study Adds?
•
Simultaneous
administration of BCG and OPV at birth prolongs the time of scar
formation.
|
References
1. Yewale V, Choudhary P, Thackar N, editors. IAP
Guide Book on Immunization. IAP Committee on Immunization 2009-2011. p.
47-52.
2. Faridi MMA, Krishnamurthy S. Abortive reaction and
time of scar formation after BCG vaccination. Vaccine.
2008;17:26:289-90.
3. Kaur S, Faridi MMA, Agarwal KN. BCG vaccination
reaction in low birth weight infants. Indian J Med Res. 2002;116:64-9.
4. Sartono E, Lisse IM, Terveer EM, Van de Sande
PJM, Whittle H, Fisker AB, et al. Oral polio vaccine influences
the immune response to BCG vaccination. PLoS One. 2010;5: e10328.
5. Lubchenco LO, Hansman C, Dressler M, Boyd E.
Intrauterine growth as estimated from live born birth weight data at 24
to 42 weeks of gestation. Pediatrics. 1963;32:793-800.
6. Infant and Young Child Feeding Counseling: A
Training Course: The 3 in 1 course-2008 Version (An Integrated Course on
Breastfeeding, Complementary feeding and Infant Feeding & HIV –
Counseling). Breastfeeding Promotion Network of India and IBFAN Asia.
7. Seth V, Kabra SK. BCG Vaccination. In:
Essentials of Tuberculosis in Children, 3rd Ed; 2006; p. 597-620.
8. Thayyil-sudan S, Kumar A, Singh M, Paul VK,
Deorari AK. Safety and efficacy of BCG vaccination in preterm babies.
Arch Dis Child. 1999;81:F-646.
9. Gupta P, Faridi MMA, Shah D, Dev G. BCG reaction
in twin newborns: Effect of zygosity and chorionicity. Indian Pediatr.
2008;45:271-7.
10. Ahmad S, Javid I. Absence of scar formation in
infants after BCG vaccination. Professional Med J. 2006;13:637-41.
11. Lakhar BB. Neonatal BCG vaccination and scar
success. Indian Pediatr. 1995; 32:1323.
12. Joshi S. BCG vaccination (immunization dialogue).
Indian Pediatr. 2000;37:332-3.
13. Sedaghatian M, Hashaem F, Hossain MM. Bacillus
Calmette-Guerin vaccination in preterm infants. Int J Tuber Lung
Dis.1998;2:679-82.
14. Fine PEM, Ponnighaus JM, Maine N. The
distribution and implication of BCG scar in Northern Malawi. Bull
World Health Organ. 1989;67:35-42.
15. Jensen KJ, Karkov HS, Lund N, Andersen A, Eriksen
HB, Barbosa AG, et al. The immunological effects of oral polio
vaccine provided with BCG vaccine at birth: A randomised trial. Vaccine.
2014;32:5949-56.
16. Sallusto F, Lenig D, Förster R, Lipp M,
Lanzavecchia A.Two subsets of memory T lymphocytes with distinct homing
potentials and effector functions. Nature. 1999;401:708-12.
|
|
|
 |
|

