|
|
|
Indian Pediatr 2015;52: 109 -114 |
 |
The Influence of Fetal Growth Restriction on
Cardiovascular Health among Adolescents in Brazil: A
Retrospective Cohort Study
|
|
PJS Alves, ACPT Henriques,
*KF
Silva, #AJM Leite,
#FEL Feitosa,
#CHM Alencar and
#FHC Carvalho
From Department of Community Health, *Faculty
of Medicine, and #Department of Maternal and Child
Health, Federal University of Ceara, Fortaleza, Brazil.
Correspondence to: Priscilla de Jesus dos Santos
Alves. Department of Community Health, Faculty of Medicine, Federal
University of Ceara. R. Prof. Costa Mendes 1608/ 5º andar, Rodolfo
Teófilo. CEP: 60430-140, Fortaleza-CE.
Email: [email protected]
Received: July 14, 2014;
Initial review: August 21, 2014;
Accepted: November 13, 2014.
|
Objective: To investigate whether fetal growth restriction is
associated with changes in cardiovascular risk factors later in life.
Design: A retrospective cohort study.
Settings: Tertiary-care hospital serving
urban population from the Brazilian Northeast.
Participants/patients: 172 adolescents aged 10-20
years were evaluated for the effects of fetal growth restriction on
anthropometric measurements, blood pressure, lipids and fasting glucose
and flow-mediated brachial artery dilatation.
Intervention: The adolescents’ birth
weight and their gestational age at birth were used to identify fetal
growth restriction according to the 10th percentile and divided between
exposed (<10th percentile) and not exposed ( ³10th
percentile). The Student-t test or the Mann-Whitney test and chi-square
were used. The significance level was considered to be 0.05.
Main Outcome Measure(s): Current Anthropometric,
metabolic and endothelial measures of subjects.
Results: The majority of the current
anthropometric, metabolic and endothelial measures did not differ
between groups. The unexposed group had a higher hip circumference (89.1
cm) and higher total cholesterol (196.4 mg/dL) than those exposed (85.4
cm, 136.9 mg/dL, respectively) (P=0.04).
Conclusions: In the sample studied, no
association was found between fetal growth restriction and changes in
cardiovascular risk factors in adolescents.
Keywords: Cardiovascular disease, Fetal growth retardation,
Gestational age, Risk factors.
|
|
S
mall for gestational age (SGA) babies presently
constitute 27% of live births worldwide [1,2]. In addition to the damage
in infancy, such as an increased risk for mortality
[3]
and cognitive impairment
[4], being born SGA is also associated with a
higher prevalence of chronic diseases in adulthood [5-9]. Explanatory
models for the association between intrauterine growth restriction and
its effect on physiological processes are based on the reduced number of
nephrons [10], altered
arterial compliance [11] or
fetal exposure to excess glucocorticoid
[12] identified in these individuals.
Evidence shows that changes, such as increased blood
pressure, can be identified early in children and adolescents who have
suffered intrauterine constraint [13-16]. On the other hand, a growing
number of studies have identified a positive association or lack of
association between fetal growth restriction and some cardiovascular
risk factors such as blood pressure [17], increased anthropometric
measurements [9], endothelial dysfunction [18]
and metabolic syndrome [19]. In low income
countries, the nutritional recovery of children with fetal growth
restriction seems to reduce morbidity and mortality [20], while in
countries with higher incomes, it is associated with a higher prevalence
of cardiovascular diseases [21].
We conducted this study to investigate whether fetal
growth restriction is associated with changes in cardiovascular risk
factors among urban individuals aged 10-20 years.
Methods
This retrospective cohort study was conducted among a
predominantly urban population in the Brazilian northeast. The Research
Ethics Committee of the Assis Chateaubriand Maternity Teaching Hospital
– Federal University of Ceará approved this study, by means of the
protocol 197.298. The study was conducted between February and August
2013 and respected all ethical and legal guidelines for research on
humans. An informed consent form was obtained from all participants or
their legal guardians.
Information on newborn weight, length and birth
conditions were taken from the hospital’s birth records, which give data
on the day and hour of the birth. It was possible to select exposed and
non-exposed to fetal growth restriction (FGR) newborns with the same
date of birth. Children whose mother’s name and medical code were
registered in these records were selected for the study, regardless of
their month of birth. FGR was defined as newborns weighing <10 th
percentile of the standard weight at birth [22].
The eligibility criteria were the absence of genetic
syndromes, cardiovascular and/or endocrine diseases and to be
healthy. Healthy subjects were understood to be participants without any
medical conditions that act directly or indirectly to increase the
cardiovascular risk. Large for gestational age newborns were excluded.
From the data recorded in the books of births,
patients with a low birth weight (<2,500 g) and normal weight (between
2,500 and 3,999 g) were identified, to search for their records and
analyze the neonatal data. The invitation to participate in this study
was made through personal visits, phone calls and letters, using the
contact data from the medical records. During the first conversation
with the adult responsible for the adolescent, the study objectives were
explained and a day was scheduled for the anthropometric and laboratory
evaluations to be carried out.
Only 58 adolescents, aged between 10 and 20 years,
with FGR, and 114 subjects with percentiles >10 th
for weight and gestational age at birth were assessed. The adolescents
were of both gender and were born at the Assis Chateaubriand Maternity
Teaching Hospital, one of the referral maternity hospitals for high-risk
pregnancies in a urban population from Ceará state, Brazil, according to
Fig.1.
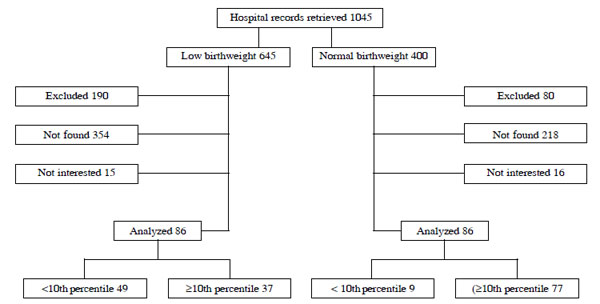 |
|
Fig. 1 Study flow diagram.
|
The initial examination included a medical and family
history, clinical interviews, followed by a fasting biochemical
assessment at the Pr Dr Eurico Litton Pinheiro de Freitas Laboratory of
Clinical and Toxicological Analysis, Federal University of Ceará (FUC -
LCTA). Venous blood samples (10 mL) were collected between 08:00 and
09:00 at the FUC-LCTA, by puncturing a vein in the forearm after fasting
for 12 hours. Vacutainer tubes containing separator gel were used to
obtain the serum. The samples were analyzed by enzymatic colorimetric
methods for glucose, total cholesterol, High Density Lipoprotein
Cholesterol (HDL-c) and triglycerides and read on a semi-automated (Labtest
Diagnostic S/A Lagoa Santa, MG, Brazil) system, following the
manufacturer’s guidelines. The Friedewald formula was used to determine
the LDL-C (Low Density Lipoprotein Cholesterol) when trigly-cerides
<400mg/dL [26]. The references of the I Guide-line for Prevention of
Atherosclerosis in Childhood and Adolescence [27] were adopted as normal
parameters.
Height was measured using a 206 model Seca
stadiometer attached to a wall. To check weight
and the total percentage of body fat an Ultra SlimW835, Wiso digital
analyzer was used. During the assessments the participants were standing
without shoes wearing light clothing. The Body Mass Index (BMI) was
calculated by dividing weight by height squared (in kilograms per square
meter) and classified according to the WHO curves [23]. The
circumference of the waist, abdomen and hips were measured using an
anthro-pometric tape measure Model T872-Wiso at the end of a gentle
expiration, taking as the reference point halfway between the lower rib
and the top of the iliac crest and umbilical scar and the largest point
of the outer hip, respectively. Moreover, the biceps fold of the right
arm was measured using a skinfold caliper model Innovare 2 Cescorf.
Systolic and diastolic blood pressure were measured
using a calibrated semi-automatic sphygmomanometer Microlife BP 3BTO-H
model, after 30 minutes of rest. Two measurements were taken at 1 minute
intervals and the average value was used for analysis. In case of a
difference of ³20
mmHg between the measurements, a new measurement was performed and the
average of the two closest measurements was used as the final result for
analysis.
Flow-mediated brachial artery dilatation (FMD):
The examinations took place between 10:30 and 12:30, in a room with
dimmed lighting and a controlled temperature (21 ºC to 24 ºC). We used a
linear probe of a Sonoace X8, Medison device with a frequency of 6-9
MHz, positioned on the medial side of the abducted right arm,
longitudinal and perpendicular to the skin, 5 cm above the antecubital
crease. The brachial artery was insonated directly below biceps and
beside the brachial muscle. The methodology developed by Cunha Filho, et
al. [24] was followed in order to verify the luminal diameter of the
brachial artery. Dilation was considered normal when
³10% [25].
Statistical analyses: The sample size was
calculated considering an alpha error of 5% and a confidence level of
80% (beta error of 20%) and, based on the occurrence of previous studies
with relative risk for complications in the long term of approximately
2.3, an "n" of 160 patients was found. The Shapiro-Wilk test was used
to test the normality of the continuous variables. The Student t-test,
Kruskall Wallis test and Mann-Whitney test were used according to the
normality of the continuous variables and Chi-square test for
categorical variables. The level of significance was set at P<0.05. The
Stata program version 12.0 was used for the statistical analysis.
Results
Web Table I shows the baseline data of the
mothers of the participants, demonstrating no differences between the
groups. Table I compares the perinatal data between the
two groups. Neonatal outcomes were less favorable in the group with FGR:
prematurity in more than 50% (P=0.01), 80% with a birth weight
less than 2,500 g (P<0.001), and shorter height and smaller head
and thoracic circumference (P<0.001).
TABLE I Perinatal Data
in the Study Population
|
Neonatal Characteristics |
Not FGR |
FGR |
|
(n=114) |
(n=56) |
|
Male |
43(37.7) |
22(37.9) |
|
Preterm (<37 wks)$ |
37(32.5) |
30(51.7) |
|
*Weight (Kg)# |
2.81(0.8) |
1.94(0.49) |
|
VLBW (<1.5 Kg)# |
6(7.1) |
12(20.7) |
|
LBW (<2.5 Kg) |
30(26.3) |
37(63.8) |
|
Underweight (2.5-2.9 Kg) |
15(13.2) |
9(15.5) |
|
Appropriate weight (3.0-3.9 Kg) |
62(54.4) |
0 |
|
*Length (m)# |
0.47(0.4) |
0.43(0.34) |
|
Ponderal index# |
|
|
|
<2.25 |
15(13.3) |
21(36.2) |
|
> 2.25 |
98(86.8) |
37(63.8) |
|
*Head circumference (cm)# |
32.9(3.5) |
31.0(2.3) |
|
*Chest circumference (cm)# |
32.1(3.8) |
28.2(3.3) |
|
Complications at birth |
71(62.8) |
42(72.4) |
|
Value in n (%) or *Mean (Standard Deviation); VLBW: very low
birth weight; LBW: low birth weight; FGR: Fetal growth
retardation # p<0.001;
$ p=0.01. |
Clinical and anthropometric data of the adolescents
was similar in the two groups except for higher hip-circumference and
higher total cholesterol levels in those without FGR (P=0.04 for
both) (Table II). No differences were noted between the
groups with regards to the brachial artery measurements (Table
III).
TABLE II Clinical and Metabolic Data in Adolescents According to Fetal Growth Restriction
|
Characteristics |
Not FGR |
FGR |
P |
|
Anthropometric variables |
|
Age (y) |
13.4 (2.8) |
12.9 (2.4) |
0.35 |
|
Weight (Kg) |
51.3 (14.6) |
46.9 (11.3) |
0.06 |
|
Height (m) |
1.55 (0.1) |
1.52 (0.1) |
0.12 |
|
BMI (kg / m2) |
21.3 (5.0) |
20.1 (3.8) |
0.20 |
|
SBP (mmHg) |
101.8 (12.6) |
101.9 (11.4) |
0.98 |
|
DBP (mmHg) |
64.6 (8.3) |
63.6 (8.1) |
0.45 |
|
AC (cm) |
75.8 (12.6) |
72.3 (10.3) |
0.12 |
|
WA (cm) |
70.0 (11.9) |
66.9 (8.1) |
0.16 |
|
HC (cm) |
89.1 (11.8) |
85.4 (9.9) |
0.04 |
|
WHR |
0.8 (0.1) |
0.8 (0.1) |
0.71 |
|
WHtR |
0.6 (0.1) |
0.5 (0.1) |
0.45 |
|
Biceps fold (mm) |
8.0 (4.3) |
7.6 (4.0) |
0.56 |
|
Body fat (%) |
28.0 (9.3) |
26.5 (9.7) |
0.32 |
|
Metabolic variables |
|
Total cholesterol (mg/dL) |
146.4 (26.0) |
136.9 (22.5) |
0.04 |
|
Triglycerides (mg/dL) |
73.5 (38.4) |
68.7 (26.1) |
0.93 |
|
HDL-c (mg/dL) |
44.2 (10.4) |
43.2 (9.7) |
0.60 |
|
LDL-c (mg/dL) |
86.6 (23.3) |
79.9 (19.7) |
0.17 |
|
VLDL-c (mg/dL) |
14.6 (7.5) |
13.7 (5.2) |
0.93 |
|
Fasting glucose (mg/dL) |
81.3 (9.1) |
79.5 (13.3) |
0.64 |
|
BMI: Body Mass Index, SBP: systolic blood pressure, DBP:
diastolic blood pressure, AC: Abdominal circumference, WC: waist
circumference; HC: hip circumference, WHR: waist to hip ratio;
WHtR: waist height ratio, LDL-C: Low-Density;
Lipoprotein; HDL-C: High-Density Lipoprotein
Cholesterol; VLDL-c: Very-Low Density Lipoprotein Cholesterol. |
TABLE IIII Brachial Artery Measurements in Adolescents According to Birthweight
|
Characteristics |
Not FGR |
FGR |
P |
|
*Basal diameter (mm) |
2.56 (0.31) |
2.58 (0.34) |
0.81 |
|
*Post-occlusion diameter (mm) |
2.86 (0.35) |
2.92 (0.37) |
0.42 |
|
*FMD, (%) |
11.96 (6.24) |
13.43 (6.41) |
0.13 |
|
Endothelial dysfunction <10%, n(%) |
41 (36) |
15 (26) |
0.18 |
|
FMD, flow-mediated dilatation of the brachial artery; *Mean
(SD). |
Discussion
The results only revealed a few anthropometric,
metabolic, and endothelial differences associated with fetal growth
restriction, specifically, hip circumference and total cholesterol, both
with lower values in FGR (P=0.04 for both). In general, those
exposed to FGR seem to be thinner and shorter, which affects other
measurements such as waist and abdominal circumference, as well as the
percentage of total fat, but without statistical differences.
This study has some limitations. The records of
neonatal and maternal data were obtained at delivery for purposes of
care and not to conduct research, leading to a lack of annotation of
some parameters. In addition, possible errors filling in the
registration can no longer be confirmed or corrected, due to the time
elapsed. As no previous measurements of the parameters analyzed in this
study had been performed, it was not possible either to identify whether
there had been rapid growth during childhood or to verify if it would
have a more significant effect than FGR, as found in some studies. Nor
was it possible to carry out the analysis excluding premature subjects
in both groups, as this would cause the loss of more than half the
patients in the group with FGR. In addition, prior to 1998, many records
were destroyed due to inadequate care, so the number of participants
over 15 years old was reduced. To reduce bias, we controlled for the
socioeconomic status. Furthermore, we have not investigated the effects
of rapid growth in children with FGR. An important feature in this study
is that socioeconomic status and the same day of birth was accounted for
and the groups were similar in maternal and family characteristics, mean
chronological age and gender. Medical registers were used to investigate
weight and height at birth in order to minimize wrong values. We used
international parameters to facilitate future comparisons with our data.
Monteiro, et al. [9], in a cohort study in
Southern Brazil, with adolescents aged 14 to 16 years found no
association between FGR and overweight/obesity among girls, whereas for
boys, the association was positive for both conditions. Another study,
which gathered data from five countries: India, Guatemala, the
Philippines and Brazil showed that the greatest above normal weight gain
at any age was related to elevated blood pressure in young adults, who
had their weight monitored after birth, at 12, 24 and at 48 months, with
the latest measurements taken on average at age 23. However, faster
weight gain in infancy did not represent a greater risk than weight gain
at other ages [30]. Some authors argue that it is not fetal restriction
or low birth weight that impact negatively on cardiovascular health, but
instead greater than expected growth, either in weight or height, also
known as catch-up growth. Furthermore, the effect of accelerated
growth itself after birth is controversial. In lower income countries,
its occurrence appears to reduce the morbidity and mortality of children
with FGR [28]; a fact not observed in countries with high incomes, where
it appears to be associated with an increased prevalence of
cardiovascular disease [21]. It is possible that other measurements
besides birth weight may be more strongly associated with adverse
cardiovascular outcomes than birth weight alone [28].
These results do not exclude the possibility of an
association between accelerated growth during the first years of life in
those with FGR and worsening risk factors. It is suggested that
prospective longitudinal studies with multiple assessments throughout
the study period are performed to verify the effect of accelerated
growth in similar populations.
Contributors: All authors have planned,
executed, construction of the research, implemented of survey, analysis
and interpretation of data and approved the final version of the
manuscript.
Funding: Financing through scholarships from the
following institutions: Ceará Foundation for Scientific and
Technological Development (Fundação Cearense de Apoio ao Desenvolvimento
Científico e Tecnológico) (FUNCAP) and the Coordination of Personnel
Improvement in Higher Education (Coordenação de Aperfeiçoamento de
Pessoal do Nível Superior) (CAPES).
Competing interests: None stated.
|
What is Already Known?
• Fetal growth
restriction is associated with some cardiovascular risk factors
such as blood pressure, increased anthropometric measurements,
endothelial dysfunction and metabolic syndrome at later ages.
What this Study Adds?
•
Only lower hip circumference and
total cholesterol in adolescence were found to be associated
with fetal growth restriction.
|
References
1. Lee ACC, Katz J, Blencowe H, Cousens S, Kozuki
N, Vogel JP, et al. National and regional estimates of term
and preterm babies born small for gestational age in 138 low-income
and middle-income countries in 2010. Lancet Glob Health.
2013;1:e26-e36.
2. WHO ECoPS. Physical status: The use of and
interpretation of anthropometry, Report of a WHO Expert Committee.
1995. Geneva: World Health Organization.
3. Black RE, Allen LH, Bhutta ZA, Caulfield LE,
de Onis M, Ezzati M, et al. Maternal and child undernutrition:
global and regional exposures and health consequences.
Lancet. 2008;371:243-60.
4. Victora CG, Adair L, Fall C, Hallal PC,
Martorell R, Richter L, et al. Maternal and child
undernutrition: consequences for adult health and human capital.
Lancet. 2008;371:340-57.
5. Barker DJP. Fetal origins of coronary heart
disease. BMJ. 1995;311:171-4.
6. Barker DJP, Osmond C, Kajantie E, Eriksson JG.
Growth and chronic disease: findings in the Helsinki Birth Cohort.
Ann Hum Biol. 2009;36:445-58.
7. Cheung YF, Wong KY, Lam BCC, Tsoi NS. Relation
of arterial stiffness with gestational age and birth weight. Arch
Dis Child. 2004;89:217-21.
8. Jafar T, Qadri Z, Islam M, Hatcher J, Bhutta
Z, Chaturvedi N. Rise in childhood obesity with persistently high
rates of undernutrition among urban school-aged Indo-Asian children.
Arch Dis Child. 2008;93:373-8.
9. Monteiro POA, Victora CG, Barros FC, Monteiro
L. Birth size, early childhood growth, and adolescent obesity in a
Brazilian birth cohort. Int J Obes. 2003;27:1274-82.
10. Dotsch J. Renal and extrarenal mechanisms of
perinatal programming after intrauterine growth restriction.
Hypertens Res. 2009;32:238-41.
11. Zanardo V, Fanelli T, Weiner G, Fanos V,
Zaninotto M, Visentin S, et al. Intrauterine growth
restriction is associated with persistent aortic wall thickening and
glomerular proteinuria during infancy. Kidney Int. 2011;80:119-23.
12. Kapoor A, Dunn E, Kostaki A, Andrews MH,
Matthews SG. Fetal programming of hypothalamo-pituitary-adrenal
function: prenatal stress and glucocorticoids. J Physiol.
2006;572:31-44.
13. Leeson CPM, Whincup PH, Cook DG, Donald AE,
Papacosta O, Lucas A, et al. Flow-mediated dilation in 9- to
11-year-old children: The influence of intrauterine and childhood
factors. Circulation. 1997;96:2233-8.
14. Belfort MB, Rifas-Shiman SL, Rich-Edwards J,
Kleinman KP, Gillman MW. Size at birth, infant growth, and blood
pressure at three years of age. J Pediatr 2007;151:670-4.
15. Donker GA, Labarthe DR, Hamst RB, Selwyn BJ,
Srinivasan SR, Wattigney W, et al. Low birth weight and serum
lipid concentrations at age 7–11 years in a biracial sample. Am J
Epidemiol. 1997;145:398-407.
16. Rodríguez-Soriano J, Aguirre M, Oliveros R,
Vallo A. Long-term renal follow-up of extremely low birth weight
infants. Pediatr Nephrol. 2005;20:579-84.
17. Fattal-Valevski A, Bassan H, Bernheim J,
Redianu B, Leitner Y, Harel S. Blood pressure values in 8-12 year
old children with a history of intrauterine growth retardation. Isr
Med Assoc J. 2011;13:480-4.
18. Rossi P, Tauzin L, Marchand E, Boussuges A,
Gaudart J, Frances Y. Respective roles of preterm birth and fetal
growth restriction in blood pressure and arterial stiffness in
adolescence. J Adolesc Health. 2011;48:520-2.
19. Vielwerth SE, Jensen RB, Larsen T, Holst KK,
Mølgaard C, Greisen G, et al. The effect of birthweight upon
insulin resistance and associated cardiovascular risk factors in
adolescence is not explained by fetal growth velocity in the third
trimester as measured by repeated ultrasound fetometry. Diabetologia.
2008 51:1483-92.
20. Victora CG, Barros FC, Horta BL, Martorell R.
Short-term benefits of catch-up growth for small-for-gestational-age
infants. Int J Epidemiol. 2001;30:1325-30.
21. Eriksson JG, Forsen T, Tuomilehto J, Osmond
C, Barker DJ. Early growth and coronary heart disease in later life:
longitudinal study. BMJ. 2001;322:949-53.
22. Pedreira CE, Pinto FA, Pereira SP, Costa ES.
Birth weight patterns by gestational age in Brazil. An Acad Bras
Cienc. 2011;83:619-25.
23. Onis M, Onyango AW, Borghi E, Siyam A,
Nishida C, Siekmann J. Development of a WHO growth reference for
school-aged children and adolescents. Bull World Health Organ.
2007;85:660-7.
24. Cunha Filho EV, Mohr C, Acauan Filho BJ,
Gadonski G, Paula LG, Antonello ICF, et al. Flow-mediated
dilatation in the differential diagnosis of preeclampsia syndrome.
Arq Bras Cardiol. 2010;94:182-6.
25. Celermajer DS, Sorensen KE, Gooch VM,
Spiegelhalter DJ, Miller OI, Sullivan ID, et al. Non-invasive
detection of endothelial dysfunction in children and adults at risk
of atherosclerosis. Lancet. 1992;340:1111-5.
26. Friedewald WT, Levy RI, Fredrickson DS.
Estimation of the concentration of low-density lipoprotein
cholesterol in plasma, without use of the preparative
ultracentrifuge. Clin Chem. 1972;18:499-502.
27. Giuliano ICB, Caramelli B, Pellanda L, Duncan
B, Mattos S, Fonseca FH. I diretriz de prevenção da aterosclerose na
infância e na adolescência. Arq Bras Cardiol. 2005;85.
28. Lucas A, Fewtrell M, Cole T. Fetal origins of
adult disease-the hypothesis revisited. BMJ. 1999;319:245.
29. Hemachandra AH, Howards PP, Furth SL,
Klebanoff MA. Birth Weight, Postnatal growth, and risk for high
blood pressure at 7 years of age: Results from the Collaborative
Perinatal Project. Pediatrics. 2007;119:e1264-e70.
30. Adair LS, Martorell R, Stein AD, Hallal PC,
Sachdev HS, Prabhakaran D, et al. Size at birth, weight gain in
infancy and childhood, and adult blood pressure in 5 low-and
middle-income-country cohorts: when does weight gain matter? Am J Clin
Nutr. 2009;89:1383-92.
|
|
|
 |
|

