|
|
|
Indian Pediatr 2014;51:
113-118 |
 |
Intranasal Clonidine vs. Midazolam as
Premedication in Children:
A Randomized Controlled Trial
|
|
Sukanya Mitra, Sunita Kazal and Lakesh K Anand
From Department of Anaesthesia and Intensive Care, Government Medical
College and Hospital, Chandigarh, India.
Correspondence: Dr Sukanya Mitra, 203-B, New Type-V Flats, Sector
24-A, Chandigarh 160023, India.
Email: drsmitra12@yahoo.com
Received: March 26, 2013;
Initial review: April 12, 2013;
Accepted: August 29, 2013.
Published online: September 05, 2013.
PII: S097475591300299
|
Objectives: To compare anxiolysis produced by intranasal clonidine
with intranasal midazolam as premedication in children undergoing
surgery.
Design: Double-blind randomized controlled study.
Setting: Tertiary-care hospital, July 2009 to
June 2010.
Patients: 60 American Society of
Anesthesiologists physical status I-II surgical patients aged 1-10 yr.
Intervention: Participants randomly allocated to
receive either intranasal clonidine 4 mcg/kg (Group I) with atropine or
intranasal midazolam 0.3 mg/kg (Group II).
Outcome measures: Primary: satisfactory
anxiolysis at 30 min after drug administration. Secondary: satisfactory
mask acceptance, times of onset of sedation and anxiolysis, drug
acceptance, level of sedation, wake-up score and side effects.
Results: All children achieved satisfactory
anxiolysis at 30 min. Group I fared significantly better than Group-II
on mask acceptance (100% in Group I vs. 80% in Group II; P=0.024),
drug acceptance (93% vs. 13%; P<0.001) and proportion of
patients with satisfactory wake-up scores (100% vs. 53%; P<0.001).
Group II patients had significantly faster onset of sedation (median 10
min vs. 15 min; P<0.05) but not that of anxiolysis
compared to Group-I (median 10 min for both groups; P>0.05). Side
effects were significantly more frequent in Group II.
Conclusions: Though intranasal midazolam produced
faster sedation, both the drugs produced satisfactory anxiolysis at 30
min.
Keywords:
Anxiolysis, Clonidine, Efficacy, Midazolam
|
|
Induction of anesthesia is a stressful and
anxiety-provoking experience for children undergoing surgery [1]. One of
the main concerns of the pediatric anesthesiologist is the appropriate
management of preoperative anxiety. This is because uncontrolled severe
preoperative anxiety and distress may lead to prolonged induction of
anesthesia and later negative postoperative behavioural sequelae [2].
Sedative premedication in general is considered to be an effective
option for reduction of preoperative anxiety in children [3].
Midazolam is by far the most commonly used sedative
premedicant [1,4], though it is far from ideal due to many shortcomings
[5,6]. Clonidine is increasingly used in pediatric population as a
sedative and analgesic because of its central
a2-adrenoceptor
agonist action [7,8]. It has been successfully used orally,
intravenously, intrathecally, epidurally and intramus-cularly in
children in a dose range of 1-5 mcg/kg [5,7,8]. The published studies on
intranasal clonidine as a premedicant in pediatric population have shown
encouraging results [9-11].
Although clonidine has been compared with midazolam
as premedication in children through the rectal [12]
and oral [13]
routes, no study directly compared intranasal clonidine
and midazolam as a premedication in the pediatric population. Thus the
present study was designed to compare the efficacy of intranasal
midazolam and intranasal clonidine to produce satisfactory levels of
anxiolysis as a premedicant for children undergoing surgery.
Methods
The study was conducted from July 2009 to June 2010.
Ethical approval for this study was provided by the Institutional Ethics
Committee. Children of either sex, in age group of 1-10 yr, of American
Society of Anesthesiologists (ASA) physical status I and II only,
scheduled to undergo minor elective surgical procedures such as
hydrocele repair, herniorrhaphy, circumcision or eye surgery were
included in this prospective randomized parallel group (with 1:1
allocation ratio) double-blind study after obtaining written informed
consent from the parents of these children and additional assent from
children over 7 years. The exclusion criteria were: children with
rhinopharyngitis or recent upper respiratory tract infection, known
allergy or hypersensitivity to clonidine or midazolam, children
requiring intravenous induction, cardiac arrhythmias, congenital heart
disease, prolonged PR interval, atrioventricular blocks, intrinsic
bradycardia, prematurity, mental retardation, raised intracranial
pressure, history of convulsions, liver and renal disease, and children
refusing to take the whole dose of premedication.
No child received any premedication before arrival in
the operating room. Patients were randomized by computer generated
random number list and randomly allocated to one of the two groups by
using coded and sealed opaque envelopes for administration of study drug
30 minutes prior to surgery. The coded syringes were prepared by a
person not involved in the study. The contents of the syringe were
unknown to the person administering the drug and the anesthetist
involved in the study. One person assessed the children during the study
period. Another administered the nasal drug and noted the drug
acceptance but was not involved in assessing anxiolysis, sedation or
mask acceptance. Baseline heart rate, SpO 2
and respiratory rate was monitored before the administration of drug.
Group I patients received 4 mcg/kg
intranasal clonidine (150 mcg/mL intravenous preparation; Clonidine
hydrochloride, Neon Laboratories Limited, India) mixed with 20 mcg/kg of
atropine. Atropine 0.6 mg/mL (Tropin, Neon Laboratories Limited, India)
was given to prevent reduction in heart rate associated with clonidine.
Group II patients received 0.3 mg/kg of midazolam (5mg/mL intravenous
preparation; Mezolam, Neon Laboratories Limited, India) using a syringe
whose needle was removed. The drugs were loaded in a graduated syringe,
and instilled in separate nostrils in 0.2 mL aliquots, with the patient
lying in semi-recumbent or supine position, till the total dose of drugs
was administered. Heart rate, respiratory rate and SpO 2
was monitored every 5 minutes after administration
of drug until transfer to operating room. Drug acceptance was recorded,
defined as crying or complaints like nasal stinging and bitter taste
after instillation of drug. The side effects of the study drugs, if any,
were also noted during the study period.
Sedation score was assessed every 5 minutes from the
administration of drug with the six-point Ramsay sedation score [14]
for maximum of 60 minutes. Anxiety was similarly
evaluated every 5 minutes by a four-point scale [15].
When an anxiolysis score of 4 or more was reached,
the child was transferred to the operating room for induction and the
time was noted. The time to reach point 4 on the anxiety scale was also
noted. If no satisfactory anxiolysis level was achieved after 60
minutes, anesthesia induction was conducted. The primary outcome measure
was proportions of patients in each group with satisfactory anxiolysis
at 30 minutes after drug administration (scores 3-4 on the relevant
scale). This primary outcome measure was selected a priori,
because this was considered to be of foremost clinical relevance in the
context of these drugs. Secondary outcome measures included times of
onset of sedation and anxiolysis, and proportion of patients with
acceptance of the drug (i.e., not crying after drug
administration), satisfactory mask acceptance (scores 3-4 on the
relevant scale), satisfactory level of sedation (scores 4-6 on the
sedation scale) and satisfactory waking up (scores 1-2 on the wake-up
scale).
A standard technique for conduct of anesthesia was
maintained for all the patients. Patients were transferred to the
operating room accompanied by one parent. After placement of routine
monitoring, anesthesia was initiated with 70% nitrous oxide in oxygen
and sevoflurane via transparent face mask kept gently on face [15,16]
and maintained with oxygen, nitrous oxide, sevoflurane and fentanyl
2mcg/kg. Behavior at awakening was evaluated with 4-point wake up score
[17].
Statistical analysis: Fisher’s Exact test was
used to compare proportions. Onset time of sedation and anxiolysis was
analyzed by Kaplan Meier survival curve and log rank test. Statistical
significance was accepted if P value was less than 0.05. All data
were analyzed using Statistical Package for Social Sciences (SPSS)15.0.
As there was no previous study directly comparing
intranasal clonidine with intranasal midazolam, a pilot study was done
using 10 patients in each group. Proportion of patients with
satisfactory anxiolysis was 80% and 100%, respectively. Using this data,
and setting alpha at 5% and power at 80%, we needed 35 patients in each
group.
Results
A total of 60 patients were enrolled, 30 in each
group (23 males in group I and 27 in group II). The demographic profiles
of the patients of two groups were similar with median (range) age of
2.5 (1-10) and 4 (1-10) years, respectively in group I and group II. The
median (range) duration of surgery was 64 (35-90) and 62 (30-80) minutes
in the two groups, respectively. The flow of patients in the study is
shown in Fig. 1.
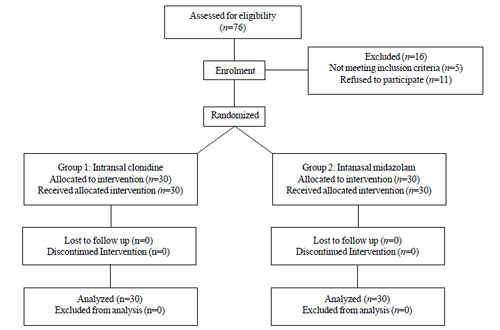 |
|
Fig.1 Flow of patients in the study
|
All the patients in both the groups developed
acceptable levels of anxiolysis (anxiety score 3-4) after 30 minutes of
drug administration. The secondary outcomes and adverse effects are
shown in Table I and Figs. 2 and 3.
TABLE I Comparison of Secondary Outcomes and Adverse Effects in The Two Groups
|
Group-I (Clonidine)N=30 (%) |
Group-II (Midazolam)N=30 (%) |
P value |
|
Drug acceptance (No crying) |
28 (93.3) |
4 (13.3) |
<0.001 |
|
†Duration of crying (s) |
0 (0-60) |
50 (0 – 120) |
<0.001 |
|
Nasal stinging |
0 |
28 (93.3) |
<0.001 |
|
Bitter taste |
0 |
15 (50.0) |
<0.001 |
|
Mask acceptance score* |
|
|
|
|
1 |
0 |
3 (10.0) |
|
|
2 |
0 |
3 (10.0) |
|
|
3 |
11 (36.7) |
14 (46.7) |
|
|
4 |
19 (63.3) |
10 (33.3) |
|
|
Satisfactory level (3-4) |
30 (100) |
24 (80.0) |
0.024 |
|
Sedation score at 30 min# |
|
|
|
|
1 |
0 |
0 |
|
|
2 |
0 |
0 |
|
|
3 |
0 |
5 (16.7) |
|
|
4 |
7 (23.3) |
15 (50.0) |
|
|
5 |
13 (43.3) |
5 (16.7) |
|
|
6 |
10 (33.3) |
5 (16.7) |
|
|
Acceptable level (4-6) |
30 (100) |
25 (83.4) |
0.052 |
|
Wake-up score$ |
|
|
|
|
1 |
10 (33.3) |
1 (3.3) |
|
|
2 |
20 (66.7) |
15 (50.0) |
|
|
3 |
0 |
13 (43.3) |
|
|
4 |
0 |
1 (3.3) |
|
|
Acceptable level (1-2) |
30 (100) |
16 (53.3) |
<0.001 |
|
*As per reference 23; #As per ref. 22, $As
per ref. 24; †median (range). |
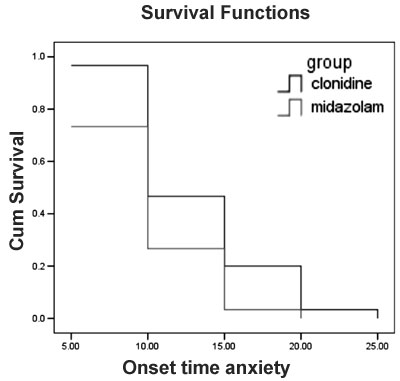 |
|
Fig. 2 Kaplan-Meier survival curve
showing onset of anxiolysis in the two groups. p = 0.7261 (Log
rank test).
|
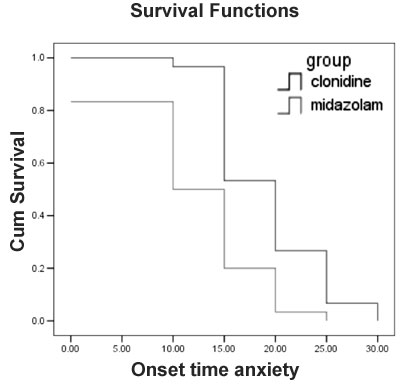 |
|
Fig. 3 Kaplan-Meier survival curve
showing onset of sedation in the two groups. p = 0.0208 (Log
rank test).
|
Crying during drug administration, median duration of
crying, complaints of nasal stinging and bitter taste were significantly
higher in group II (Table I). A higher number of patients
in Group I had a mask acceptance score 3-4.
Postoperatively, all the patients in Group I were
either calm and cooperative or could be easily consoled (i.e.,
wake-up scores 1-2) as compared to 53% of patients in Group II (P
< 0.001).
Discussion
This randomized controlled trial compared intranasal
clonidine and intranasal midazolam as premedication in children
undergoing elective surgery found satisfactory anxiety score in both
groups. Mukherjee, et al. [16] found that the onset of anxiolysis
after clonidine premedication was 15.8±2.6 minutes whereas Almenrader,
et al. [9] reported 23.3±17.2 minutes for the onset of anxiolysis,
that was longer than reported in our study. Kogan, et al. [15]
found that the maximal anxiolysis was achieved at 20 minutes after
intranasal midazolam administration. However, others have reported that
intranasal midazolam provided maximal sedation and anxiolysis within 10
minutes after administration [18,19]. The results of our study are
broadly in line with the previous studies, with the exception of one
[9].
Findings on various drug effect related parameters
have varied markedly across various studies. This variation might be due
to several factors such as drug dose, preparation, exact mode of
administration (single, repeated, patient position, etc.),
observer-related factors, patient-related factors, state of nasal
mucosa, preoperative information and experience, and even cultural and
environmental differences in experiencing and reporting some outcomes.
There is a practical limit to the total volume of the drug that can be
instilled through the nasal route. Inadvertent swallowing of the drug
and subsequent gastric absorption are other potential drawbacks. It has
been shown that direct transport of clonidine from the nasal mucosa to
systemic circulation can be erratic and unpredictable [11]. Further,
atropine was co-administered intranasally in the clonidine group. Nasal
atropine has been shown to reduce nasal secretions and mucociliary
clearance [20,21], which might have favored nasal clonidine absorption
in our study.
As regards the secondary outcome measures, drug
acceptance was better in clonidine group than midazolam. Midazolam,
either directly or because of its acidic pH, may be responsible for
nasal mucosal irritation, thus causing low acceptance [6,15].
Significantly more patients in the clonidine group
than in the midazolam group accepted mask satisfactorily. Mask
acceptance can be an important composite marker signifying a combination
of anxiolysis, lack of fear, drug tolerability and the resultant
cooperativeness. Steal induction could be performed in four (13.3%) of
the patients in clonidine group as compared to three (10%) in midazolam
group. Almenrader, et al. [9] found that steal induction was
possible in 60% of the patients whereas Mukherjee, et al. could
perform it in 20% of the patients [16]. Although patients were
well-sedated, steal-induction could not be performed as patients were
waking up during transfer. It is possible that Almenrader, et al.
achieved steal induction in 60% cases because they induced children in
the parents’ arms in a dimmed and quiet operating room [9].
The onset of sedation was significantly faster in
midazolam group as compared to clonidine group in this study but both
the groups achieved acceptable sedation levels at 30 min. The onset of
sedation after clonidine premedication in our study is consistent with
their findings [9,16] but faster compared to few other reports
[18,19,22,23]. In contrast, a recent publication compared two dose
strengths of an aerosol preparation of nasal clonidine with placebo in a
double-blind randomized trial and found that only 55% of the children
receiving the higher dose (7-8 µg/kg) were adequately sedated at 30 min
after administration of the aerosol [24]. The variations in these study
results might be because of several factors mentioned above. It is an
interesting and important area for future research.
Finally, patients in clonidine group had
significantly better wake up score than midazolam group. Previous
reports [9,16] also found that the majority of the patients were either
calm and cooperative, or could be easily consoled postoperatively when
clonidine was used as premedicant. Other authors also report that
clonidine produces more effective early postoperative analgesia, reduces
the incidence of postoperative nausea vomiting and shivering, and causes
attenuation of postoperative delirium when compared to midazolam and
thus produces better wake up score [5,8]. This is consistent with the
results obtained in our study. Further, midazolam, a benzodiazepine,
causes anterograde and retrograde amnesia, and this has been suggested
to be a potential mechanism for causing poorer wake-up score and early
postoperative agitation in the midazolam group [5].
We did not study the cognitive functions of the
children before and after receiving the drugs. This may be considered a
limitation of the study, though our primary focus was on efficacy. Other
limitations include a sub-optimal sample size lack of a placebo control
group and lack of generalizability of the findings in children
undergoing emergency surgery. Further, preoperative anxiety was measured
by a previously used scale [15] but not compared with other validated
scales [25]. However, these limitations should not invalidate the main
conclusions from this study.
In conclusion, intranasal clonidine has been shown to
produce comparable level of sedation and effective anxiolysis as nasal
midazolam after 30 minutes, but with a better mask acceptance and
recovery profile.
Contributors: SM: conception and design,
interpretation of results, critical inputs to manuscripts writing; SK:
study design, data collection, analysis and interpretation, and
manuscript writing; LA: study design, data interpretation and critical
inputs to manuscript writing. All authors approved the final version of
manuscript.
Funding: None; Competing interests: None
stated.
References
1. McCann ME, Kain ZN. The management of preoperative
anxiety in children. Anesth Analg. 2001;93:98-105.
2. Kain Z, Mayes L. Anxiety in children during the
perioperative period. In: Borestein M, Genevro J, Mahwah NJ,
eds. Child Development and Behavioral Pediatrics. Mahwah: Lawrence
Erlbaum Associates, 1996. p. 85-103.
3. Kain Z, Mayes L, Wang S, Caramico LA, Hofstadter
MB. Parental presence during induction of anesthesia vs. sedative
premedication: which intervention is more effective? Anesthesiology.
1998;89:1147-56.
4. Kain ZN, Magnus LC, Bell C, Weisman S, Hofstadter
MB, Rimar S. Premedication in United States - a status report.
Anesth Analg. 1997;84:427-32.
5. Bergendahl H, Lonnqvist PA, Eksborg S. Clonidine
in paediatric anaesthesia: review of literature and comparison with
benzodiazepines for premedication. Acta Anaesthesiol Scand.
2006;50:135-43.
6. Lonnqvist PA, Habre W. Midazolam as premedication:
is the Emperor naked or just half-dressed? Pediatr Anesth.
2005;15:263-5.
7. Basker S, Singh G, Jacob R. Clonidine in
paediatrics – a review. Indian J Anaesth. 2009;53:270-80.
8. Dahmani S, Brasher C, Stany I, Golmard J, Skhiri
A, Bruneau B, et al. Premedication with clonidine is superior to
benzodiazepines. A meta-analysis of published studies. Acta Anaesthesiol
Scand. 2010;54:397-402.
9. Almenrader N, Passariello M, Coccetti B, Haiberger
R, Pietropaoli P. Steal induction after clonidine premedication:
Comparison of oral and nasal route. Pediatr Anesth.
2007;17:230-4.
10. Stella MJ, Bailey AG. Intranasal clonidine as a
premedicant: three cases with unique indications. Pediatr Anesth.
2008;18:71-3.
11. Almenrader N, Larsson P, Passariello M, Haiberger
R, Pietropaoli P, Lönnqvist PA, et al. Absorption
pharmacokinetics of clonidine nasal drops in children. Pediatr Anesth.
2009;19:257-61.
12. Bergendahl HT, Lönnqvist PA, Eksborg S, Ruthström
E, Nordenberg L, Zetterqvist H, et al. Clonidine vs. midazolam as
premedication in children undergoing adenotonsillec-tomy: a prospective,
randomized, controlled clinical trial. Acta Anaesthesiol Scand.
2004;48:1292-1300.
13. Almenrader N, Passariello M, Coccetti B,
Haiberger R, Pietropaoli P, et al. Premedication in children: a
comparison of oral midazolam and oral clonidine. Pediatr Anesth.
2007;17:1143-9.
14. Ramsay MA, Savege TM, Simpson BR, Goodwin R.
Controlled sedation with alphaxalone-alphadolone. BMJ. 1974;2:656-9.
15. Kogan A, Katz J, Efrat R, Eidelman LA.
Premedication with midazolam in young children: a comparison of 4 routes
of administration. Pediatr Anesth. 2002;12:685-9.
16. Mukherjee S, Ray M, Ray A, Khanra M, Mandal PK,
Pal R. Clonidine premedication for paediatric patients: a comparison of
the oral and nasal route. J Anaesth Clin Pharmacol. 2010;26:319-22.
17. Yuen VM, Hui TW, Irwin MG, Yuen MK. A comparison
of intranasal dexmedetomidine and oral midazolam for premedication in
pediatric anesthesia: a double blind randomized controlled trial. Anesth
Analg. 2008;106:1715-21.
18. Karl HW, Rosenberger JL, Larach MG, Ruffle JM.
Transmucosal administration of midazolam for premedication of patients –
comparison of nasal and sublingual route. Anesthesiology.
1993;78:885-91.
19. Bhakta P, Ghosh BR, Roy M, Mukherjee G.
Evaluation of intranasal midazolam for preanaesthetic sedation in
paediatric patients. Indian J Anaesth. 2007;51:111-6.
20. Georgitis JW. Nasal atropine sulfate: efficacy
and safety of 0.050% and 0.075% solutions for severe rhinorrhea. Arch
Otolaryngol Head Neck Surg. 1998;124:916-20.
21. Takeuchi K, Suzumura E, Majima Y, Sakakura Y.
Effect of atropine on nasal mucociliary clearance. Acta Otolaryngol.
1990;110:120-3.
22. Griffith N, Howell S, Mason DG. Intranasal
midazolam for premedication of children undergoing day-case anaesthesia:
comparison of two delivery systems with assessment of intra-observer
variability. Br J Anaesth. 1998;81:865-9.
23. Malinvosky JM, Populare C. Premedication with
midazolam in children: effects of intranasal, rectal and oral route on
plasma midazolam concentration. Anaesthesia. 1995;50:351-4.
24. Larsson P, Eksborg S, Lönnqvist PA. Onset time
for pharmacologic premedication with clonidine as a nasal aerosol: a
double-blind, placebo-controlled, randomized trial. Ped Anesth.
2012;22:877-83.
25. Kain ZN, Mayes LC, Cicchetti DV, Bagnall
AL, Finley JD, Hofstadter MB. The Yale Preoperative Anxiety Scale: how
does it compare with a ‘‘gold standard’’? Anesth Analg. 1997;85:783-8.
|
|
|
 |
|

