|
|
|
Indian Pediatr 2014;51:
105-111 |
 |
Fractional Exhaled Nitric Oxide in Children
with Acute Exacerbation of Asthma
|
|
Dinesh Raj, Rakesh Lodha, Aparna Mukherjee, Tavpritesh Sethi, *Anurag
Agrawal and Sushil Kumar Kabra
From Division of Pediatric Pulmonology, Department of
Pediatrics, All India Institute of Medical Sciences, Ansari Nagar, New
Delhi 110029 and *Molecular Immunogenetics, CSIR-Institute of Genomics
and Integrative Biology, New Delhi 110 007, India.
Correspondence to: Dr Rakesh Lodha, Additional
Professor, Department of Pediatrics, All India Institute of Medical
Sciences, Ansari Nagar, New Delhi 110 029, India.
Email: rlodha1661@gmail.com
Received: May 16, 2013;
Initial review: July 03, 2013;
Accepted: August 07, 2013.
Published online: September 05, 2013.
PII: S097475591300498
|
Objective: To determine whether fractional exhaled nitric oxide
(FENO) has a utility as a diagnostic or predictive maker in acute
exacerbations of asthma in children.
Design: Analysis of data collected in a pediatric
asthma cohort.
Setting: Pediatric Chest Clinic of a tertiary
care hospital
Methods: A cohort of children with asthma was
followed up every 3 months in addition to any acute exacerbation visits.
Pulmonary function tests (PFT) and FENO were obtained at all visits. We
compared the FENO values during acute exacerbations with those at
baseline and those during the follow up.
Results: 243 asthmatic children were enrolled
from August 2009 to December 2011 [mean (SD) follow up - 434 (227)
days]. FENO during acute exacerbations was not different from FENO
during follow up; however, FENO was significantly higher than personal
best FENO during follow up (P < 0.0001). FENO during acute
exacerbation did not correlate with the severity of acute exacerbation (P=0.29).
The receiver operating characteristics curve for FENO as a marker for
acute exacerbation had an area under the curve of 0.59. Cut-off of 20
ppb had a poor sensitivity (44%) and specificity (68.7%) for acute
exacerbation.
Conclusions: FENO levels during acute
exacerbation increase from their personal best levels. However, no
particular cut off could be identified that could help in either
diagnosing acute exacerbation or predicting its severity.
Keywords: Acute exacerbation, Asthma, FENO, Nitric oxide.
|
|
N
itric oxide (NO) was first measured in exhaled
air in 1991 [1] and association with asthma was reported in 1993 [2].
Fractional exhaled nitric oxide (FENO) is a marker of asthma, and high
levels correlate with ongoing eosinophilic inflammation [3]. FENO levels
typically come down with inhaled corticosteroids (ICS) in a
dose-dependent manner [4,5]. In case of loss of asthma control, FENO
increases [6], and there are some data to suggest that serial monitoring
of FENO can help in titrating corticosteroid doses [7] and predicting
exacerbation [8].
American Thoracic Society guidelines recommend the
use of exhaled NO in management of asthmatics, especially in asthma with
eosinophilic inflammation and in predicting response to corticosteroids
[9]. However, the role of FENO in asthma at present is limited to
diagnosis of eosinophilic airway inflammation, monitoring of airway
inflammation, and likelihood of steroid responsiveness. FENO has been
studied in acute exacerbation, but has been limited to reproducibility
of measurements, emergency department disposition, and response to
corticosteroids [10-12]. Most of these studies have been done in
Caucasian children and studies from the Indian subcontinent are lacking.
There is a need to evaluate the utility of FENO in
acute exacerbations as it may reflect the extent of airway inflammation.
We conducted this study to determine the utility of FENO measurements in
acute exacerbations.
Methods
This study was conducted in the Pediatric Chest
Clinic of the All India Institute of Medical Sciences (AIIMS), New
Delhi, which is a tertiary care teaching hospital. We are following a
cohort of pediatric asthma patients (up to 18 yrs age) since August
2009. Study protocol was approved by Ethics committees of AIIMS, New
Delhi and CSIR-IGIB, New Delhi..
Written informed consent was taken from the
parents/guardian. The diagnosis and treatment of asthma was based on the
Global Initiative for Asthma (GINA) guidelines [13] by a pediatric
pulmonologist. The patients were followed up every 3 months, symptom
diary was maintained, and control was assessed as per GINA guidelines.
On each visit, lung function measurements (spirometry and impulse
oscillometry) were performed and FENO levels were obtained. Blood was
collected at enrollment for peripheral eosinophil counts. Therapy was
modified, if required, on the basis of clinical features and spirometry.
In case of appearance of symptoms of acute
exacerbation, they contacted the research team (led by a pediatrician)
and a visit was scheduled. Children who were not able to perform
spirometry or FENO were not included in the analysis.
The patients with acute exacerbation were evaluated
and managed in the Pediatrics department by the study team. Acute
exacerbation was defined as recent increase in asthma symptoms requiring
hospital visit and treatment with salbutamol and/or steroids [14].
Initial evaluation included history and physical examination (including
pulse oximetry). Severity of acute asthma was assessed using pulmonary
score [15]. Child was initially evaluated in clinic, and subsequently
managed in emergency room in case of moderate and severe acute
exacerbation. Child underwent FENO measurement followed by spirometry.
Patients were managed according to acute asthma guidelines [14]. Apart
from asthma management, compliance and technique was checked and
re-emphasized at each visit.
FENO measurement: FENO measurement was done using
NIOX MINO (Aerocrine AB, Solna, Sweden) in accordance with ATS
guidelines [9]. FENO was measured at the time of enrolment, on each
follow up visit every 3 months and on each breakthrough visit that was
assessed to be acute exacerbation. In mild to moderate exacerbations,
FENO measurement was done in clinic before bronchodilator therapy. In
case of severe exacerbation, child was managed in emergency room and
FENO was done once child was stable. All measurements were performed on
the same equipment and by similarly trained team-members, ensuring
minimal technical variability.
Spirometry was performed after FENO measurement was
done. Spirometry was done using portable spirometer (Superspiro MK2,
Micro Medical Ltd, UK) in all children in accordance with ATS
standards [16].
Pulmonary score is a validated measure of asthma
severity for children with acute asthma exacerbation [15]. Each
parameter is rated on a 0-3 scale, with a maximum total score of 9.
Mild, moderate, and severe acute exacerbations were defined as pulmonary
score of 0-3, 4-6, and 7-9, respectively.
Skin prick testing (SPT) was done using 12
aeroallergens during the follow up of the cohort. Saline was taken as
negative control and histamine was used for positive control. Patients
were not on antihistaminics for at least 48 hours preceding the test.
The twelve allergens tested were rice grain dust, wheat threshing dust,
housefly, female cockroach, dog dander, house dust mite (Dermatophagoides
farinae), Curvularia lunata, Aspergillus tamari,
Alternaria tenius, Prosopis juliflora, Cynodon dactylon,
and Holoptelea integrifolia. Allergens were obtained from All
Cure Pharma Pvt Ltd, Bahadurgarh, Haryana. Test was considered positive
if wheal in any of the allergens was 3 mm or more than the negative
control. Child was considered atopic if he demonstrated positive result
to one or more allergen, and non-atopic if he had a negative SPT.
Statistical analysis: Data were entered
using Microsoft Access. Statistical analysis was performed using Stata
9.0 statistical software (Stata Corp., College Station, TX, USA). We
identified each patient’s minimum FENO value during follow up (personal
best). We determined the difference in FENO measured during acute
exacerbation as compared to the follow up values and also as compared to
the personal best for each patient. The distribution of FENO values was
skewed to the left, so it was reported as median (IQR). The differences
between FENO at various time points were analyzed using Wilcoxon
signed-rank test. The difference in FENO across more than two groups was
analyzed using Kruskal Wallis test. We constructed receiver operating
characteristics (ROC) curves to assess the ability of FENO to predict
acute exacerbation and we hypothesized FENO of 20 ppb as a cut off for
acute exacerbation.
We divided patients on the basis of baseline FENO
into three sub-groups (low, intermediate, and high) i.e., <20
ppb, 20 to 35 ppb, and >35 ppb using ATS guidelines [9]. We then
assessed the exacerbation rates and also various FENO values in these
three categories. Correlation between FENO and pulmonary score was done
using Spearman correlation test. A P value of <0.05 was
considered significant.
Results
The cohort of 243 children (76% males) was enrolled
between August 2009 to December 2011. Forty-six children out of 243 were
steroid naïve at baseline. Baseline characteristics of the cohort are
given in Table I. Eosinophil count was available in 80
children. Absolute eosinophil count was 327/mm 3
(IQR: 216-609) and 264/mm3
(IQR: 196-800) in non-atopic and atopic children, respectively (P=0.99).
We had a total of 1183 FENO measurements from the children enrolled.
TABLE I Baseline Characteristics
|
Characteristics |
|
|
Number of patients |
243 |
|
Mean (SD) age, mo
|
99 (41.7) |
|
Males, n (%) |
180 (76.1) |
|
Duration of follow up, days; mean (SD) |
434 (227) |
|
Residence |
|
|
Rural, n (%) |
50 (20.7) |
|
Urban, n (%) |
184 (76.35) |
|
Urban slum, n (%) |
7 (2.9) |
|
Family history
|
|
|
Asthma, n (%) |
127 (52.7) |
|
Asthma, nasal allergy, or eczema, n (%) |
161 (66.8) |
|
Exposure to tobacco smoke at home, n (%) |
95 (39.6) |
|
Baseline asthma severity
|
|
|
Intermittent asthma, n (%) |
25 (10.3)
|
|
Mild persistent asthma, n (%)
|
120 (49.4)
|
|
Moderate persistent asthma, n (%) |
94 (38.7)
|
|
Severe persistent asthma, n (%) |
4 (1.7) |
|
Absolute eosinophil count, cells/µL (n=95) |
655 (935) |
|
Atopy (skin prick testing, n=180) |
|
|
Positive to at least one allergen, n (%) |
100 (55.6) |
|
Positive to more than one allergen, n (%) |
68 (37.8) |
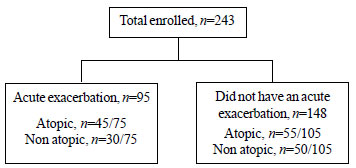 |
|
Fig. 1 Flow chart depicting acute
exacerbation during follow up and atopic status.
(Atopic status was assessed using SPT in
children above 5 years of age).
|
A flow chart of enrolled children depicting acute
exacerbation and atopic status is given in Fig. 1. One
hundred and seventy four exacerbations were diagnosed in 95 patients
(39.1%) during the study period (Table II). Forty seven
patients (19.4%) had two or more exacerbations. FENO measurements were
available in 143 exacerbations. The overall median number of FENO
measure-ments in children who had an exacerbation was 8 (range 3 - 12).
TABLE II Characteristics of Study Subjects During Follow-up
|
Characteristics |
|
|
Total number of exacerbations, n |
174 |
|
Exacerbation rate, per child per year |
0.75 |
|
Children with at least 1 exacerbation, n (%) |
95 (39.1) |
|
Children with at least 2 exacerbations, n (%) |
47 (19.4) |
|
Severity of exacerbation (n=166) |
|
|
Mild (Pulmonary score 0-3) |
139 (83.7%) |
|
Moderate (Pulmonary score 4-6) |
26 (15.7%) |
|
Severe (Pulmonary score >6) |
1 (0.6%) |
|
FENO (ppb), median (IQR) |
|
|
Baseline (n=185) |
15 (9-26) |
|
Personal best (n=218) |
8 (5-12) |
|
During exacerbation (n=143) |
17.7 (12-25.3) |
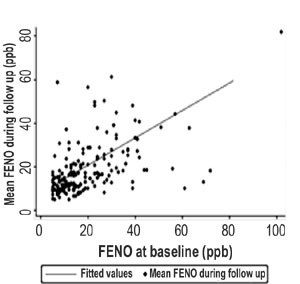 |
|
Fig. 2 FENO
measurements of enrolled children at baseline and during follow
up
|
The baseline and mean follow up FENO measurements are
shown in Fig. 2. Median (IQR) FENO during follow up and
baseline was 14.4 ppb (8.2-21.3) and 15 ppb (10-26), respectively. We
looked at the difference in FENO at baseline and during acute
exacerbation in children who suffered an exacerbation. Median (IQR)
baseline FENO at enrolment was 15 ppb (9-26) while that during
exacerbation was 17.7 ppb (12-25.3), the difference was not
statistically significant (P=0.064). We identified each patient’s
minimum FENO value during follow up (personal best); the FENO during an
acute exacerbation [17.7 ppb (12-25.3)] was significantly higher than
the personal best FENO [8 ppb (5-12), P<0.0001]. The median
difference in FENO values 3 months after the baseline measurement of
FENO was 0.5 ppb in children who did not have an exacerbation in the
first 3 months of follow up.
FENO values increased above the personal best value
for an individual patient in 114/143 episodes (79.7%). The median (IQR)
personal best FENO (n=94) and FENO during acute exacerbation (n=84)
were 7.5 (5-12) and 17.7 (12-25.3), respectively (P<0.001). The
median change between personal best FENO and acute exacerbation FENO was
8.5 ppb (IQR: 4.3-13 ppb) (n=84). The median percentage increase
in FENO (from personal best to acute exacerbation) was 121% (IQR: 46.4 -
200). The median absolute change in FENO (personal best to acute
exacerbation FENO) was 5 ppb (IQR: 2-12), and 9 ppb (IQR: 5-13) in
non-atopic and atopic children, respectively (P=0.10).
Table III shows the FENO values at different time points in
children according to the severity of asthma.
TABLE III NIH Asthma Severity and FENO at Various Time Points
|
Asthma severity |
FENO at enrolment,
|
Minimum FENO,
|
Acute exacerbation
|
|
ppb; median (IQR) |
ppb;median (IQR) |
FENO, ppb; median (IQR) |
|
Intermittent, n=4 |
13 (10-16) |
5
|
11
|
|
Mild persistent, n=80 |
15 (10-23) |
8 (5-11) |
21 (13.5-26) |
|
Moderate persistent, n=108 |
14 (9-25) |
8 (5-11) |
15 (12-22) |
|
Severe persistent, n=24 |
20 (12-36) |
12 (8- 20) |
20 (14-29) |
|
P value |
0.46 |
0.0015 |
0.34 |
We divided patients on the basis of baseline FENO
into three sub groups (low, intermediate, and high)
i.e., <20, 20
to 35, and >35 ppb. The median FENO during follow up, personal best
FENO, and FENO during acute exacerbation were higher in children with
higher baseline FENO values (Table IV). However, the
exacerbation rates per child per year were similar in the 3 categories
i.e. 0.81, 0.66, and 1.22 in the low, intermediate, and high FENO
category, respectively (P=0.76).
TABLE IV FENO at Various Time-points in Different FENO Subgroups
|
FENO category |
FENO at enrolment, |
FENO during follow up, |
Personal best FENO, |
FENO during Acute
|
|
ppb; median (IQR) |
ppb; median (IQR) |
ppb; median (IQR) |
exacerbation, ppb;
|
|
|
|
|
median (IQR) |
|
FENO <20 |
11 (8-14), n=117 |
12 (9.3-17), n=105 |
7 (5-10), n=117 |
14 (11-21), n=45 |
|
FENO ³20 to <35 |
26 (23-30), n=45 |
22.3 (16-32.9), n=42 |
12 (9-21), n=45 |
26.3 (18-35), n=19 |
|
FENO ³35 |
42 (39-57), n=23 |
27.1 (18.3-38), n=23 |
13 (10-18), n=23 |
21.7 (19.5-23.5), n=7 |
|
P value |
0.0001 |
0.0001 |
0.0001 |
0.0012 |
As only one severe exacerbation was observed, so for
the purpose of analysis, moderate and severe exacerbations were taken as
one group. Median FENO during acute exacerbation was 18 ppb (IQR: 12-26)
and 14 ppb (IQR: 10-25) in mild and moderate exacerbation respectively (P=0.39).
Pulmonary score did not correlate with acute exacerbation FENO (r=0.1,
Spearman correlation, P=0.29).
Sixty-three (75%) children had an FENO
³20 ppb during
exacerbation. Using ROC curve, a cut off of 20 ppb had a sensitivity of
44% and a specificity of 68.7%, with an area under curve (AUC) of 0.59.
Cut off of 15 ppb had a sensitivity of 57.3% and a specificity of 53.5%.
Cut off of 25 ppb had a sensitivity of 27.3% and a specificity of 78.7%.
The AUC was higher for children with a baseline FENO
<20 ppb (AUC=0.64) as compared to those with baseline FENO
³20 to <35 ppb
(AUC=0.52) and FENO ³35
ppb (AUC=0.36) [P=0.0009] suggesting better discriminatory value
of FENO for exacerbation in children with a lower FENO at baseline.
Discussion
We evaluated the utility of FENO in children with
acute exacerbation of asthma in this cohort study. This is the first
study to have compared FENO levels during follow up to FENO levels
during acute exacerbation. The personal best FENO was significantly
lower than FENO during acute exacerbation.
Poor asthma control can lead to asthma exacerbation,
so we expected increase in FENO during exacerbation from personal best
levels. While in around 68% children, the FENO during acute exacerbation
was at least 5 ppb higher than the personal best FENO, the difference
was either less than 5 ppb or even negative (i.e., best personal
FENO less than acute exacerbation FENO) in the remaining one-third.
Thus, FENO’s utility in predicting the presence of acute exacerbation is
limited.
FENO is influenced by a number of factors. Atopy
[17], viral infections [18], allergen exposure [19, 20] and concomitant
rhinitis [21] are known to increase FENO. FENO is known to decrease with
smoke exposure [22], post-spirometry [23], and corticosteroid treatment
[4,5]. In our study, almost all children were on anti-inflammatory
treatment. There is some evidence that height correlates positively with
FENO [24]. Smoke exposure and air pollution are particularly important
to our patients in Delhi, India, where air quality is notoriously poor.
It is likely that at one point in time there are multiple factors which
play role in precipitating an acute exacerbation, and these factors have
a complex effect on airway NO metabolism. Thus, in a pragmatic clinical
setting, analogous to that seen by respiratory physicians in developing
nations like India, FENO has probably only limited clinical utility such
as predicting response to inhaled steroids.
The ROC curve for FENO as a marker for acute
exacerbation had an AUC of 0.59. Cut of off 20 ppb had poor sensitivity
and specificity for diagnosis of acute exacerbation. An FENO value of 16
ppb had a sensitivity of 56.6% and a specificity of 58.3%, which was the
best combination of sensitivity and specificity. No study has so far
evaluated FENO cut offs for acute exacerbation. It is evident from our
study that FENO cut off values have poor sensitivity and specificity in
predicting acute exacerbations.
Atopy is associated with high FENO, airway
hyperresponsiveness, and deterioration with response to allergen
exposure. We were interested in knowing whether atopic children
demonstrate a higher increase in FENO during acute exacerbation than
non-atopic children, which was not evident in the results (P=0.10).
The possible explanation being that most children were on
anti-inflammatory medications during acute exacerbations, and the
complex interplay of NO metabolism affected by various factors.
We investigated the association between severity of
acute exacerbation and FENO concentrations. We used pulmonary score [14]
to assess the severity of acute exacerbation. Majority of the acute
exacerbations were of mild-moderate severity. Only one severe
exacerbation was seen in our cohort. The reason behind this could be
rigorous follow up, telephonic interactions, and home visits to ensure
adequate adherence to the controller treatment regimen which may have
prevented severe exacerbations.
We did not find any association between FENO and
severity of acute exacerbation. Kwok et al measured FENO during acute
exacerbation in children aged 2-18 years of age, and reported no
difference in their median FENO concentration, regardless of their
severity of acute asthma [10].
It is logical to think that the more severe the
exacerbation, the more inflammation should be evident. Airway caliber
also affects FENO concentrations, and decrease in airway caliber has
been shown to decrease FENO [25, 26]. This is one of the reasons which
possibly could negate the increase in FENO in moderate-severe
exacerbations, apart from the other confounders like, age, height, viral
infections, allergen exposure etc.
Not much work has been done on evaluation of FENO in
the setting of acute exacerbation. Kwok et al found measurement of FENO
difficult for a large proportion of children with acute asthma. In their
study FENO measurement could be obtained in only 68% children [10].
Gill, et al. found poor reproducibility of FENO measurements
obtained in emergency department patients with acute asthma
exacerbations [11]. However, Baptist, et al. showed acceptable
intraclass correlation coefficient and coefficient of variation values
(0.98 and 9.42%, respectively) for reproducibility [12]. With the advent
of portable hand held NO analyzer, it is possible to measure FENO not
only in clinic setting but also in the emergency department, but the
utility of FENO in acute exacerbation setting is probably limited.
Asthma is now increasingly being recognized to be a
heterogeneous disease constituting of several inflammatory phenotypes.
FENO is a surrogate marker of eosinophilic inflammation in asthmatic
children. Other phenotypes like neutrophilic and pauci-granulocytic are
unlikely have high FENO. Our study enrolled heterogeneous children with
asthma and assessed the utility of FENO in acute exacerbations. In our
study, it is possible that children with non-eosinophilic inflammation
had near normal FENO even during an inflammation. The heterogeneity of
patients (steroid naïve, intermittent, mild persistent, moderate
persistent, and severe persistent) could be one of the reasons of
inability of FENO to predict exacerbation. We did not evaluate viral
infection as a cause for acute exacerbation as it is known to increase
FENO. The personal best FENO value was taken as minimum of the follow up
values. The observed personal best FENO value may not have been true for
children with fewer visits.
To conclude, FENO levels during acute
exacerbation increase from their minimum follow up levels. However, no
appropriate cut off could be identified which could help diagnosing
acute exacerbation. The FENO values did not correlate with the severity
of acute exacerbation. It appears that FENO measurement may add little
to the diagnosis of acute exacerbation of asthma in children. Diagnosis
of acute exacerbation should be based on history, clinical examination,
and spirometry and rising FENO (from personal best to acute
exacerbation) may be taken as supportive evidence.
Contributors: RL, SKK, and AA: were
involved in conception and design of the study; DR, AM, and TS: were
involved in data generation; DR, RL, AM, TS, SKK, and AA; were involved
in analysis and interpretation of data; DR, RL, AM, TS, SKK, and AA:
were involved in preparation and critical revision of the manuscript.
All authors approved the final version of the manuscript.
Funding: The study was funded by a grant from
CSIR-IGIB; Competing interests: None stated.
|
What is Already Known?
• FENO, a marker of eosinophilic inflammation
is recommended for monitoring of airway inflammation.
• The role of FENO in the setting of acute
exacerbations has not been established.
What This Study Adds?
• FENO measurements add little to the diagnosis of acute
exacerbation of asthma in children.
|
References
1. Gustafsson LE, Leone AM, Persson MG, Wiklund NP,
Moncada S. Endogenous nitric oxide is present in the exhaled air of
rabbits, guinea pigs and humans. Biochem Biophys Res Commun. 1991;
181:852-7.
2. Alving K, Weitzberg E, Lundberg JM. Increased
amount of nitric oxide in exhaled air of asthmatics. Eur Respir J. 1993;
6:1368-70.
3. Jatakanon A, Lim S, Kharitonov SA, Chung KF,
Barnes PJ. Correlation between exhaled nitric oxide, sputum eosinophils,
and methacholine responsiveness in patients with mild asthma. Thorax.
1998;53:91-5.
4. Kharitonov SA, Donnelly LE, Montuschi P, Corradi
M, Collins JV, Barnes PJ. Dose-dependent onset and cessation of action
of inhaled budesonide on exhaled nitric oxide and symptoms in mild
asthma. Thorax. 2002;57:889-96.
5. Jones SL, Herbison P, Cowan JO, Flannery EM,
Hancox RJ, McLachlan CR, et al. Exhaled NO and assessment of
anti-inflammatory effects of inhaled steroid: dose-response
relationship. Eur Respir J. 2002;20:601-8.
6. Jones SL, Kittelson J, Cowan JO, Flannery EM,
Hancox RJ, McLachlan CR, Taylor DR. The predictive value of exhaled
nitric oxide measurements in assessing changes in asthma control. Am J
Respir Crit Care Med. 2001;164:738-43.
7. Pijnenburg MW, Bakker EM, Hop WC, De Jongste JC.
Titrating steroids on exhaled nitric oxide in children with asthma: a
randomized controlled trial. Am J Respir Crit Care Med. 2005;172:831-6.
8. Gelb AF, Flynn Taylor C, Shinar CM, Gutierrez C,
Zamel N. Role of spirometry and exhaled nitric oxide to predict
exacerbations in treated asthmatics. Chest. 2006; 129:1492-9.
9. Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh
MW, Lundberg JO, et al. American Thoracic Society Committee on
Interpretation of Exhaled Nitric Oxide Levels (FENO) for Clinical
Applications. An official ATS clinical practice guideline:
interpretation of exhaled nitric oxide levels (FENO) for clinical
applications. Am J Respir Crit Care Med. 2011;184:602-15.
10. Kwok MY, Walsh-Kelly CM, Gorelick MH. The role of
exhaled nitric oxide in evaluation of acute asthma in a pediatric
emergency department. Acad Emerg Med. 2009;16:21-8.
11. Gill M, Walker S, Khan A, Green SM, Kim L, Gray
S, et al. Exhaled nitric oxide levels during acute asthma
exacerbation. Acad Emerg Med. 2005;12:579-86.
12. Baptist AP, Sengupta R, Pranathiageswaran S, Wang
Y, Ager J. Evaluation of exhaled nitric oxide measurements in the
emergency department for patients with acute asthma. Ann Allergy Asthma
Immunol. 2008;100:415-9.
13. Global Strategy for Asthma Management and
Prevention, Global Initiative for Asthma (GINA) 2011. Available from:
http://www.ginasthma.org/.
14. Saharan S, Lodha R, Kabra SK. Management of
status asthmaticus in children. Indian J Pediatr. 2010;77:1417-23.
15. Smith SR, Baty JD, Hodge D 3rd. Validation of the
pulmonary score: an asthma severity score for children. Acad Emerg Med.
2002;9:99-104.
16. Miller MR, Hankinson J, Brusasco V, Burgos F,
Casaburi R, Coates A, et al. Standardisation of spirometry.
ATS/ERS Task Force. Eur Respir J. 2005;26:319-38.
17. Silvestri M, Sabatini F, Spallarossa D, Fregonese
L, Battistini E, Biraghi MG, et al. Exhaled nitric oxide levels
in non-allergic and allergic mono- or polysensitised children with
asthma. Thorax. 2001;56:857-62.
18. Kharitonov SA, Yates D, Barnes PJ. Increased
nitric oxide in exhaled air of normal human subjects with upper
respiratory tract infections. Eur Respir J. 1995;8:295-7.
19. Pedrosa M, Barranco P, López-Carrasco V, Quirce
S. Changes in exhaled nitric oxide levels after bronchial allergen
challenge. Lung. 2012;190:209-14.
20. Bodini A, Peroni D, Loiacono A, Costella S,
Pigozzi R, Baraldi E, et al. Exhaled nitric oxide daily
evaluation is effective in monitoring exposure to relevant allergens in
asthmatic children. Chest. 2007;132:1520-5.
21. Linhares D, Jacinto T, Pereira AM, Fonseca JA.
Effects of atopy and rhinitis on exhaled nitric oxide values - a
systematic review. Clin Transl Allergy. 2011;1:8.
22. de la Riva-Velasco E, Krishnan S, Dozor AJ.
Relationship between exhaled nitric oxide and exposure to low-level
environmental tobacco smoke in children with asthma on inhaled
corticosteroids. J Asthma. 2012;49:673-8.
23. Silkoff PE, Wakita S, Chatkin J, Ansarin K,
Gutierrez C, Caramori M, et al. Exhaled nitric oxide after
beta2-agonist inhalation and spirometry in asthma. Am J Respir Crit Care
Med. 1999;159:940-4.
24. Franklin PJ, Turner SW, Le Souëf PN, Stick SM.
Exhaled nitric oxide and asthma: complex interactions between atopy,
airway responsiveness, and symptoms in a community population of
children. Thorax. 2003;58:1048-52.
25. García-Río F, Ramírez M, Mediano O, Lores V, Rojo
B, Villasante C, et al. Exhaled nitric oxide and airway caliber
during exercise-induced bronchoconstriction. Int J Sports Med.
2006;27:905-10.
26. de Gouw HW, Hendriks J, Woltman AM, Twiss IM,
Sterk PJ. Exhaled nitric oxide (NO) is reduced shortly after
bronchoconstriction to direct and indirect stimuli in asthma. Am J
Respir Crit Care Med. 1998;158:315-9.
|
|
|
 |
|

