|
|
|
Indian Pediatr 2013;50:
209-213 |
 |
Effect of Probiotics on Allergic Rhinitis in
Df, Dp or Dust-Sensitive Children: A Randomized Double Blind
Controlled Trial
|
|
Teng-Yi Lin, Chia-Jung Chen *,
Li-Kuang Chen#, Shu-Hui Wen$,
and Rong-Hwa Jan†
From the Department of Laboratory Medicine and
†Pediatrics, Buddhist Tzu Chi General Hospital; and *Department of
Nursing, #Institute of Medical Sciences, and $Institute of Public
Health, College of Medicin; Tzu Chi University; Hualien, Taiwan.
Correspondence to: Rong-Hwa Jan, Department of
Pediatrics, Buddhist Tzu Chi General Hospital, #707, Section 3,
Chung-Yang Road, Hualien, Taiwan. [email protected]
Received: July 12, 2011;
Initial review: August 17, 2011;
Accepted: April 30, 2012.
Published online: 2012, June 10.
PII:S097475591100603-1
|
|
Objective: To study, we examined the effect of
Lactobacillus salivarius on the clinical symptoms and medication use
among children with established allergic rhinitis (AR).
Design: Double blind, randomized,
controlled trial.
Setting: Hualien Tzu-Chi General
Hospital.
Methods: Atopic children with
current allergic rhinitis received 4 × 109 colony forming units/g of
Lactobacillus salivarius (n=99) or placebo (n=100)
daily as a powder mixed with food or water for 12 weeks. The SCORing
Allergic rhinitis index (specific symptoms scores [SSS] and symptom
medication scores [SMS]), which measures the extent and severity of AR,
was assessed in each subject at each of the visits - 2 weeks prior to
treatment initiation (visit 0), at the beginning of the treatment (visit
1), then at 4 (visit 2), 8 (visit 3) and 12 weeks (visit 4) after
starting treatment. The WBC, RBC, platelet and, eosinophil counts as
well as the IgE antibody levels of the individuals were evaluated before
and after 3 months of treatment.
Results: The major outcome,
indicating the efficacy of Lactobacillus salivarius treatment,
was the reduction in rhinitis symptoms and drug scores. No significant
statistical differences were found between baseline or 12 weeks in the
probiotic and placebo groups for any immunological or blood cell
variables.
Conclusions: Our study demonstrates that
Lactobacillus salivarius treatment reduces rhinitis symptoms and
drug usage in children with allergic rhinitis.
|
|
Allergic rhinitis (AR) is a common childhood
disease that often persists into adulthood. The prevalence of childhood
allergic disease has increased dramatically in recent decades in many
parts of the world, including Taiwan [1]. The prevalence of reported
symptoms of AR in Taiwanese children aged 6-8 and 13-15 years has been
reported to be 29.8% and 18.3%, respectively [1]. The causes of AR are
not well understood, but sensitization to food proteins may play a role.
Children who are atopic and develop dermatitis are at a significantly
increased risk of developing atopic asthma and rhinitis in later
childhood [2]. This immune response includes both IgE antibodies and
helper T cells type 2 (Th2), which are thought to contribute to
inflammation in the respiratory tract. Moreover, sensitization to indoor
allergens (eg, dust mites, cats, and dogs) is strongly associated with
allergic rhinitis.
Probiotics are products or preparations containing
viable numbers of microorganisms that are able to modify the host’s
microflora, thereby producing beneficial health effects [3].
Lactobacilli are considered to induce reactions involving Th1 cells and
to improve allergic diseases. Two lines of argument have provided the
framework for studies on the relationship between bowel flora and
allergic disease. First, lower counts of Enterococci and
Bifidobacteria in infancy have been found in atopic vs.
non-atopic children and these differences precede sensitization [4, 5].
The early colonization of the bowel with probiotic bacteria such as
Enterococci and Bifidobacteria are hypothesized to more
effectively mature the gut mucosal immune system and promote tolerance
to non-bacterial antigens. Secondly, increased gut permeability may lead
to increased exposure to food antigens, which has been associated with
atopic dermatitis (AD) [6]. Probiotics may decrease gut permeability
thereby decreasing systemic exposure to food antigens.
Isolauri, et al. [7] have previously reported
an improvement in the SCORing Atopic Dermatitis (SCORAD) index in
milk-allergic infants with mild AD following probiotic-supplemented
hydrolyzed whey formula [8]. Recently, Rosenfeldt, et al. [8],
using a cross-over study design, demonstrated an improvement in the
SCORAD index in older children with AD who were treated with probiotics;
however, the improvement was only significant is allergic patients.
Lactobacillus paracasei may improve the quality of life of
adolescents with perennial allergic rhinitis [9, 10]. The effect of
probiotics on rhinitis, remain controversial [11, 12]. Studies have
demonstrated that oral administration with L. salivarius prior to
and during allergen sensitization and airway challenges leads to a
suppression of various features of the asthmatic phenotypes, including
specific IgE production, airway inflammation, and development of airway
hyperresponsiveness in an animal model [13]. Therefore, it is worth
while examining whether L. salivarius could improve allergic
symptoms in humans. We examined the effect of probiotic treatment on
atopic children with rhinitis. Various specific clinical and immune
parameters were assessed in allergen-sensitive patients before and after
treatment, and were compared with those of untreated (UT)
allergen-sensitive patients.
Methods
The study was conducted in the pediatric clinic of
Hualien Tzu Chi General Hospital between February and December 2009.
Children aged between 6 to 12 years; history of perennial allergic
symptoms for at least 3 years; positive skin prick test (SPT) for Dp, Df
or dust, and Unicap system (Pharmacia Diagnostics, Uppsala, Sweden)
positivity for Dp, Df, or dust (more than class 1) were recruited in the
study. Patients who had previously been treated with immunotherapy, and
those with recurrent respiratory tract and infectious diseases, were
excluded. This study was approved by the Research Ethics Committee of
the Hualien Tzu-Chi General Hospital and informed consent was obtained
from all subjects.
Randomization was performed by doctors, who were not
involved in this study design. All of the enrolled patients were
randomly assigned to the L. salivarius group or the placebo group
according to computer-generated permuted-block randomization. The
selected patients were randomized into two groups (consisting of 120 UT
patients and 120 patients treated with probiotic) taking into account
age, sex, medication scores, type and importance of ocular symptoms
(itching, redness, or weeping), nasal symptoms (sneezing, rhinorrhea,
itching or nasal blockage), and lung symptoms (cough, sputum, dyspnea,
or wheezing). The treatment group received 4 × 10 9
colony forming units/g of Lactobacillus salivarius PM-A0006
supplied by ProMD Biotech Co., Ltd. The control group received a placebo
consisting of microcrystalline cellulose that looked and tasted the same
as the probiotics. All patients who met the eligibility criteria were
randomized into either the probiotic-treated group or the control group.
The powder (500 mg) was given once daily mixed in drink or food. A small
number of older children (>10 years) took the powder as an opaque
capsule. The viability of the probiotic was tested monthly. Both
subjects and investigators were blind to the treatment groups. Study
duration was 12 weeks, followed by a 7 month observational phase to
observe disease manifestations. There were five scheduled visits: 2
weeks before starting the treatment (visit 0), at the beginning of the
treatment (visit 1), then 4 weeks (visit 2), 8 weeks (visit 3), and 12
weeks (visit 4) after treatment was initiated. Parents received two
phone calls during the treatment period to check on patient progress and
compliance (6 and 9 weeks after the beginning of the treatment). At each
visit the severity of the child’s AR was evaluated using the specific
symptoms scores (SSS) and symptom medication scores (SMS) [14,15].
All parents completed a questionnaire (visit 0) about
AR and the allergic disease history of their child, the family’s history
of allergic diseases, and any current oral or topical medication
currently in use. During the 12 week study parents were asked to
complete a weekly diary of medication use, health problems, and the
presence and severity of AR in the child to aid recall for the
questionnaire at the study visits. A final questionnaire was completed
at the last office visit, which included previous medication usage,
other allergic diseases, and changes in life style or housing during the
study.
Both the treated and UT patients maintained a weekly
diary of allergic symptoms during the antigen exposure period. Specific
symptoms scores (SSS) were recorded for nasal blockage, nasal itching,
sneezing, rhinorrhea, eye irritation and watering, wheezing, cough, and
asthma. Symptom medication scores (SMS) were calculated from patient
diaries, as described previously [14,15]. During the study, all patients
received same medications in the whole period according to individual
allergic status and allowed to take the following medications if
required. Scores were calculated based on drugs used (0.5 points for
each dose of nasal corticosteroids and 2 points for each dose of
antihistamine). Patients were instructed to use local steroids plus
antihistamines only if their symptoms did not improve. Patients were
also asked to report each administration or variation of the initial
drug therapy in the diary. Patients were also instructed to stop their
medication at least 7 days before blood sampling. At each time point
(visit 0, 1, 2, 3, and 4) of the study, a patient self-evaluation was
completed. Each patient was also asked for his/her overall evaluation of
the treatment based on categories of symptoms gravity.
Blood samples were taken before and after treatment,
to examine total IgE, peripheral blood cell counts, and blood eosinophil
counts.
Of the 240 randomized patients, 21 from the probiotic
group and 20 from the placebo group did not complete the study and
another 106 patients (44 probiotic and 49 placebo) dropped out of the
blood trial due to sampling difficulties and withdrawal of consent.
Fig. 1 shows the relevant patient flow chart.
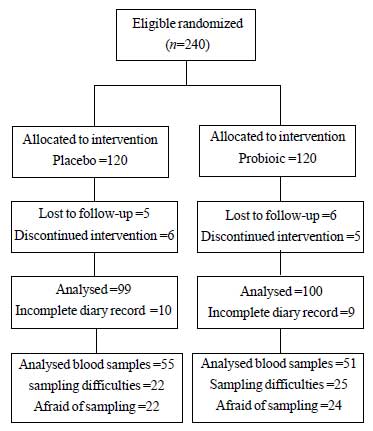 |
|
Fig.1 CONSORT diagram of the study.
|
Statistical analysis: Statistical analysis was
performed using paired and unpaired Student’s t-tests, as appropriate. A
p-value of less than 0.05 was considered statistically significant. All
analyses utilized SPSS 13.0 Statistical Software.
Results
A total of 240 (120 boys) age-matched
Dermatophagoides pteronyssinus (Dp), Dermatophagoides farinae
(Df), or dust-sensitive patients with perennial rhinitis and/or rhinitis
plus mild asthma were recruited from February to March 2009. The
two groups did not differ in terms of demographic variables, age, body
weight, gender, family history, medication scores, and allergic
symptoms. A total of 199 out of the 240 enrolled patients (82.9%)
completed the study in the year 2009. All patients were included in the
safety analysis. The demographic information is shown in Table
I.
TABLE I Baseline Characteristics of the Study Population
|
|
Placebo |
Probiotics groups |
|
N=100 |
N=99 |
|
Age (y) |
8.0 (2.1) |
8.0 (1.9) |
|
Body weight (kg) |
27.6 (4.3) |
27.0 (4.9) |
|
Gender (M/F) |
63/37 |
59/40 |
|
*Family history n
|
64
|
70
|
|
Rhinitis alone, n
|
51 |
52 |
|
Rhinitis + asthma, n |
48 |
46 |
|
Duration of disease (y) |
3.8 (0.5) |
3.8 (0.3) |
|
Mean Specific eye scores |
4.1 (0.9) |
4.3 (0.8) |
|
Mean Specific nasal scores |
8.8 (1.1) |
8.7 (1.2) |
|
Mean Specific lung scores |
8.3 (1.2) |
8.2 (1.1) |
|
Drug scores |
2.8 (1.5) |
2.9 (1.6) |
|
WBC (103/mm3) |
8.64 (2.8) |
8.49 (2.5) |
|
Eosinophils (%) |
5.36 (4.1) |
4.87 (3.8) |
|
IgE (IU/mL) |
543.38 (154.2) |
572.07 (153.2) |
|
Values are mean (SD) unless
indicated; *of allergic disease. |
Results for the three-month follow-up were based on
the patient self-evaluation scores obtained at treatment onset and after
4, 8, and 12 weeks of treatment. Study duration was 12 weeks, followed
by a 7 month observational phase to observe disease manifestations. The
SSS of Lactobacillus salivarius-treated at 8 and 12 weeks were
significantly reduced in comparison with those UT patients, specifically
for eye and nose symptom scores (eye scores: 1.0 ± _–0.5
vs. 2.4 ± –0.9 at 8 weeks and 0.6 ± –0.3 vs. 2.1 ± –0.7 at 12 weeks,
P=0.001 and 0.000; nasal scores: 5.1 ± –0.9 vs
6.5 ± –1.2 at 8 weeks and 3.1 ± –0.8 vs 5.1 ± –1.5 at 12 weeks,
P=0.001 and 0.000) (Table II a, b).
In Lactobacillus salivarius-treated patients a significant
reduction in lung SSS was not observed (Table II c). The
effect of probiotics on drug use was determined by analyzing the drug
score for allergic disease at visit 1, 2, 3, and 4. There was a
statistically significant change in medication scores for rhinitis at
visit 4 between the Lactobacillus salivarius-treated group and UT
group (2.4±_0.9 vs. 2.8 ±_1.1 , P=0.006) (Table II d).
TABLE II Specific Symptom- and Drug-Scores
|
Visit
|
Placebo |
Probiotics group |
P value |
|
Specific eye symptom scores |
|
|
|
|
1 |
4.1 (0.9) |
4.0 (0.8) |
0.262 |
|
2 |
3.0 (0.7) |
3.2 (0.9) |
0.125 |
|
3 |
2.4 (0.90) |
1.0 (0.5) |
0.001* |
|
4 |
2.1 (0.70) |
0.6 (0.3) |
0.000* |
|
Specific Nasal Symptom Scores
|
|
|
|
|
1 |
8.8 (1.1) |
8.7 (1.2) |
0.583 |
|
2 |
5.8 (2.2) |
5.1 (1.5) |
0.052 |
|
3 |
6.5 (1.2) |
5.1 (0.9) |
0.001* |
|
4 |
5.1 (1.5) |
3.1 (0.) |
0.000* |
|
Specific Lung Symptom Scores
|
|
|
|
|
1 |
8.3 (1.2) |
8.2 (1.1) |
0.363 |
|
2 |
3.5 (0.9) |
3.5 (1.0) |
0.885 |
|
3 |
2.3 (0.7) |
2.2 (0.5) |
0.236 |
|
4 |
2.4 (1.4) |
2.1 (0.5) |
0.06 |
|
Specific Medication Scores
|
|
|
|
|
1 |
2.8 (1.5) |
2.9 (1.6) |
0.769 |
|
2 |
2.7 (0.6) |
2.6 (0.5) |
0.737 |
|
3 |
2.9 (1.0) |
2.6 (0.8) |
0.083 |
|
4 |
2.8 (1.1) |
2.4 (0.9) |
0.006* |
|
There were four scheduled visits: at the beginning of the
treatment (visit 1), then 4 weeks (visit 2), 8 weeks (visit 3)
and 12 weeks (visit 4) after starting the treatment. All values
in mean (SD). * p<0.05 (Probiotics vs placebo group at each
visit).
|
Due to sampling difficulties, blood was only
collected from 106 patients (44.2%). There was no difference between the
groups in blood and immunologic profile level before the study.
Blood cell counts, total IgE, and blood eosinophil counts were not
statistically different between visit 4 and 1 in Lactobacillus
salivarius-treated or UT groups (Table III).
TABLE III Immunologic and Blood Cell Parameters
|
Group |
Variable |
visit 1 to visit 4 |
|
Mean difference (SD) |
|
|
|
Placebo (n=51) |
WBC |
1.14 |
(2.60) |
|
Eosinophil |
-0.93 |
(4.27) |
|
IgE |
-150.5 |
(632.76) |
|
Probiotics (n=55) |
WBC |
1.05 |
(2.11) |
|
Eosinophil |
0.72 |
(4.54) |
|
|
IgE |
40.34 |
(392.55) |
|
P value >0.05 for all comparisions
(visit 1 vs visit 4) |
Discussion
The aim of this study was to evaluate the effects of
a probiotic on the clinical response to allergens in Dp, Df or
dust-sensitive patients. To the best of our knowledge, there have been
no published double-blind, randomized, placebo-controlled trials
examining the effect of Lactobacillus salivarius on atopic
disease in patients with rhinitis. The major results, indicating the
efficacy of Lactobacillus salivarius treatment, was the reduction
in rhinitis symptoms and drug scores. We had also performed a study to
determine the clinical significance. Most healthy children did not
experience any medicine problem related to allergic symptoms, and nasal
and ocular SSS of the healthy participants were similar to the
Lactobacillus salivarius-treated patients’ at 8 or 12 weeks (data
not shown). Taken together, these observations shows that subjective
symptoms are good parameters to assess the condition of allergic
rhinitis. Even though a significant dropout rate (17.1%) was observed in
the current study, the mean values for SSS and SMS in the remaining
group members were similar to those of patients who dropped out. When
examined after 3 months of probiotic treatment, the Lactobacillus
salivarius-treated group reported reduced nasal and eye symptoms
compared with the UT group.
The currently reported findings are compatible with
previously published in vitro results [16-18]. The consumption of
Lactobacillus salivarius strains induces a significant increase
in IL-10 production. IL-10 cytokine can downregulate the production of
Th1 cytokines and induce the development of regulatory T cells [19, 20].
Therefore, the Lactobacillus salivarius strain acts as an immuno-modulator
with anti-inflammatory effects in the regulation of the response to
antigen challenge in allergic disease.
No difference was found in specific immune and blood
parameters between the probiotic and placebo group. This result is in
line with previous studies [21-23]. Several studies have indicated that
T-regulatory cells play an important role in regulating
allergen-specific inflammatory responses. CD4+
CD25+ Foxp3+
cells are recruited into both lungs and draining lymph nodes and can
suppress allergen-induced mucous hypersecretion, airway eosinophilia and
hyperresponsiveness [24-27]. In addition, the natural resolution of an
allergic airway response to Der p1 in mice was shown to be dependent on
CD4+ CD25+
Foxp3+ cells that appear in
the lungs and drain mediastinal lymph nodes following an airway
challenge. Recently, studies revealed that L. salivarius AH102
did not alter T-regulatory cell number in animal model tested [28]. The
exact mechanism for the effects of this strain of probiotics on the
regulatory mechanisms of the immune responses in humans is not as yet
clearly defined. Further studies are needed to clarify this point.
In conclusion, we found that a marked reduction of
the symptom scores was observed during treatment with Lactobacillus
salivarius, with differences in anti-allergic drug intake in
patients. The magnitude of the reduced symptom scores is a clinically
important issue to investigate the application of probiotics.
Funding: Supported by grants from Hualien Tzu-chi
Hospital; Competing interests: None stated.
References
1. Kao CC, Huang JL, Ou LS, See LC. The prevalence,
severity and seasonal variations of asthma, rhinitis and eczema in
Taiwanese schoolchildren. Pediatr Allergy Immunol. 2005;16:408-15.
2. Krishna MT, Mavroleon G, Holgate ST. Essentials of
Allergy. London: Martin Dunitz; 2001: 107-27.
3. Havenaar R, Huis in’t Veld JHJ. Probiotics: a
general view. The lactic acid bacteria. In: Wood BJB, Ed. The
Lactic Acid Bacteria in Health and Disease. London: Elsevier Applied
Science. 1992;1:151–71.
4. Bjorksten B, Naaber P, Sepp E, Mikelsaar M. The
intestinal microflora in alergic Estonian and Swedish 2-year-old
children. Clin Exp Immunol. 1999;29:342-6.
5. Kirjavainen P, Arvola T, Salminen S, Isolauri E.
Aberrant composition of gut microbiota of allergic infant: a target of
bifidobacterial therapy at weaning. Gut. 2002;51:51-5.
6. Pike MG, Heddle RJ, Boulton P, Turner MW, Atherton
DJ. Increased intestinal permeability in atopic eczema. J Invest
Dermatol. 1986;86:101-4.
7. Isolauri E, Arvola T, Sutas Y, Moilanen E,
Salminen S. Probiotics in the management of atopic eczema. Clin Exp
Allergy. 2000;30:1604-10.
8. Rosenfeldt V, Benfeldt E, Nielsen SD, Michaelsen
KF, Jeppesen DL, Valerius NH, et al. Effect of probiotic
Lactobacillus strains in children with atopic dermatitis. J Allergy Clin
Immunol. 2003;111:389-95.
9. Peng GC, Hsu CH. The efficacy and safety of
heat-killed Lactobacillus paracasei for treatment of perennialallergic
rhinitis induced by house-dust mite. Pediatr Allergy Immunol.
2005;16:433-8.
10. Wang MF, Lin HC, Wang YY, Hsu CH. Treatment of
perennial allergic rhinitis with lactic acid bacteria. Pediatr Allergy
Immunol. 2004;15:152-8.
11. Ciprandi G, Vizzaccaro A, Cirillo I, Tosca A.
Bacillus clausii effects in children with allergic rhinitis. Allergy.
2005;60:702-10
12. Aldinucci C, Bellussi L, Monciatti G, Passŕli GC,
Salerni L, Passŕli D, et al. Effects of dietary yoghurt on
immunological and clinical parameters of rhinopathic patients. Eur J
Clin Nutr. 2002;56:1155-61
13. Li CY, Lin HC, Hsueh KC, Wu SF, Fang SH. Oral
administration of Lactobacillus salivarius inhibits the allergic airway
response in mice Can J Microbiol. 2010;56:373-9.
14. La Rosa M, Ranno C, Andrč C, Carat F, Tosca MA,
Canonica GW. Double-blind placebo-controlled evaluation of
sublingual-swallow immunotherapy with standardizaed Parietaria Judaica
extract in children with allergic rhinoconjunctivitis. J Allergy Clin
Immunol. 1999;104:425-32.
15. Cosmi L, Santarlasci V, Angeli R, Liotta F, Maggi
L, Frosali F, et al. Sublingual immunotherapy with
Dermatophagoides monomeric allergoid down-regulates allergen-specific
immunoglobulin E and increases both interferon-g- and
interleukin-10-production. Clin Exp Allergy. 2006; 36:261-72.
16. Niers LE, Hoekstra MO, Timmerman HM, van Uden NO,
de Graaf PM, Smits HH, et al. Selection of probiotic bacteria for
prevention of allergic diseases: immunomodulation of neonatal dendritic
cells. Clin Exp Immuno. 2007;149:344-52.
17. Di´az-Ropero MP, Marti´n R, Sierra S, Lara-Villoslada
F, Rodríguez JM, Xaus J, et al. Two Lactobacillus strains,
isolated from breast milk, differently modulate the immune response. J
Appl Microbiol. 2007;102:337-43.
18. Drago L, Nicola L, Iemoli E, Banfi G, De Vecchi
E. Strain-dependent release of cytokines modulated by Lactobacillus
salivarius human isolates in an in vitro model. BMC Res Notes.
2010;3:44.
19. D’Andrea A, Aste-Amezaga M, Valiante NM, Ma X,
Kubin M, Trinchieri G. Interleukin 10 (IL-10) inhibits human lymphocyte
interferon gamma-production by suppressing natural killer cell
stimulatory factor/IL-12 synthesis in accessory cells. J Exp Med.
1993;178: 1041-8.
20. Groux H, Bigler M, de Vries JE, Roncarolo MG.
Interleukin-10 induces a long-term antigen-specific anergic state in
human CD4+ T cells. J Exp Med. 1996;184:19-29.
21. Ishida Y, Nakamura F, Kanzato H, Sawada D,
Yamamoto N, Kagata H, et al. Effect of milk fermented with
Lactobacillus acidophilus strain L-92 on symptoms of Japanese cedar
pollen allergy: a randomized placebo-controlled trial. Biosci Biotechnol
Biochem. 2005;69:1652-60.
22. Tamura M, Shikina T, Morihana T, Hayama M,
Kajimoto O, Sakamoto A, et al. Effects of probiotics on allergic
rhinitis induced by Japanese cedar pollen: randomized double-blind,
placebo-controlled clinical trial. Int Arch Allergy Immunol.
2007;143:75-82.
23. Xiao JZ, Kondo S, Yanagisawa N, Takahashi N,
Odamaki T, Iwabuchi N, et al. Effect of probiotic Bifidobacteriu
mlongum BB536 in relieving clinical symptoms and modulating plasma
cytokine levels of Japanese cedar pollinosis during the pollen season: a
randomized double-blind, placebo-controlled trial. J Investig Allergol
Clin Immunol. 2006;16:86-93.
24. Leech MD, Benson RA, De Vries A, Fitch PM, Howie
SE. Resolution of Der p1-induced allergic airway inflammation is
dependent on CD4+ CD25+Foxp3+ regulatory cells. J Immunol.
2007;179:7050-8.
25. McGlade JP, Gorman S, Zosky GR, Larcombe AN, Sly
PD, Finlay-Jones JJ, et al. Suppression of the asthmatic
phenotype by ultraviolet b-induced, antigen-specific regulatory cells.
Clin Exp Allergy. 2007;37:1267-76.
26. Strickland DH, Stumbles PA, Zosky GR, Subrata LS,
Thomas JA, Turner DJ, et al. Reversal of airway
hyperresponsiveness by induction of airway mucosal CD4+ CD25+regulatory
T cells. J Exp Med. 2006;203:2649-60.
27. Wu K, Bi Y, Sun K, Xia J, Wang Y, Wang C.
Suppression of allergic inflammation by allergen-DNA-modified dendritic
cells depends on the induction of Foxp3+ regulatory T cells. Scand J
Immunol. 2008;67:140-51.
28. Lyons A, O’Mahony D, O’Brien F, MacSharry J,
Sheil B, Ceddia M, et al. Bacterial strain-specific induction of
Foxp3+ T regulatory cells is protective in murine allergy models. Clin
Exp Allergy. 2010;40:811-9.
|
 |
|

