|
Interrupted aortic arch
(IAA) is the most frequently
associated major lesion (23%) in patients with
aortopulmonary window (APW), while APW can be
detected in only 5% of patients with IAA. To our knowledge, 44
patients with associated IAA and APW were reported. Only a few
associated major cardiac malformations, such as aortic valve
atresia, anomalous origin of left coronary artery and pulmonary
artery sling, were described in these patients, but complete
atrioventricular septal defect (CAVSD) has not been reported
yet. The diagnosis of this unusual triad was made by
transthoracic echocardiography and confirmed intraoperatively
and after autopsy.
Case Report
A male newborn, weighting 3.280 kg, was
admitted in our Institution on his 4th
day of life. He was moribund, dispneic, tachycardic and
cyanotic. He was nondysmorphic on evaluation. The liver was
palpable 4cm below the right costal margin. Peripheral pulses
were not palpable. The cardiac auscultation disclosed a gallop
rhythm and a grade 2/6 ejection systolic murmur. The
electrocardiogram showed a normal sinus rhythm, right axis
deviation, right atrial enlargement, right ventricular
hypertrophy, and nonspecific changes in ST segment. The chest
X-ray demonstrated gross cardiomegaly and increased
pulmonary vascular markings. Blood gas analysis revealed
metabolic acidosis. He was ventilated and managed medically with
prostaglandin E, dobutamin and dopamine. After overnight
stabilization, the repair of the cardiovascular defects was
performed. Fatal outcome occurred in the early postoperative
course.
Echocardiogram revealed situs solitus,
atrioventricular and ventriculoarterial concordance. The
left-sided superior caval vein drained to the right atrium via
enlarged coronary sinus. Right ventricle was well developed, and
left ventricle was border sized with AV valve index (left AV
valve/total AV valve area) estimated as 0.4. There was a huge
CAVSD with single left antero-laterally papillary muscle and
common atriventricular valve attached to the crest of the
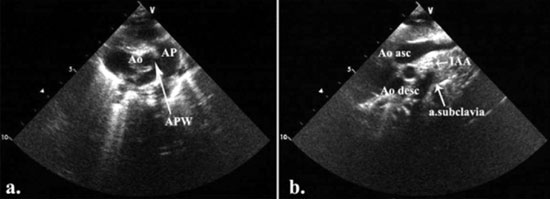
ventricular septum by chordae (Fig. 1a). Color
Doppler demonstrated moderate regurgitation on the
atrioventricular valve. The large aortopulmonary communication
was observed just above tricuspid and nonstenotic aortic valve
(APW- type 1). The relatively small ascending aorta was giving
rise right and left common carotid arteries. The left subclavian
artery was noticed arising from descending aorta just before the
patent ductus arteriosus (IAA – type B) (Fig. 1b).
 |
|
Fig. 1 (a) The defect in the
aortopulmonary septum was seen in parasternal
short-axis. Two separate aortic and pulmonary valve were
present. Ao-aorta; AP-pulmonary artery; APW-aortopulmonary
window. (b) Suprasternal long-axis view demonstrated the
interruption of the aortic arch between left common
carotid and left subclavian artery. Ao asc-ascending
aorta; Ao desc-descending aorta; IAA-interrupted aortic
arch.
|
Post sternotomy, a hypoplastic and poorly
ventilated left lung was immediately noted, which did not
respond adequately to manual hyperventilation. Left persistent
superior caval vein was dominant. Aortic arch was interrupted
after the left carotid artery. Resection of ductus arteriosus
and ligation of the left subclavian artery was performed in deep
hypothermic cardiopulmonary bypass with mobilization of the
descending aorta. The APW was 10 mm in diameter. A transection
of the APW was done and the descending aorta anastomosed
directly to the aortic part of the APW. The pulmonary artery was
reconstructed with autologous pericardium. Cardio-pulmonary
bypass was reinstituted. Due to the border sized left ventricle,
we decided to delay the complete repair of the CAVSD. After the
first cessation of the cardiopulmonary bypass and initial
promising hemodynamics with moderate inotropic support of 10
µg/kg/min of dopamine, the child developed sudden pulmonary
edema with severe blood stasis in the left lung. Reinstitution
of cardiopulmonary bypass and prolonged circulatory support gave
no effect. Low cardiac output and severe left lung blood stasis
led to the lethal outcome. The diagnosis was re-confirmed after
autopsy.
Discussion
The associations between IAA and CAVSD, as
well as between APW and CAVSD were reported previously [1,2].
IAA is present in approximately 1.3% and APW in only 0.2% of
patients with congenital heart disease [3-5]. CAVSD comprises
5%-8% of all congenital heart defects and most frequently is
associated with trisomy 21, but there is considerable evidence
of genetic heterogeneity.
Sporadic reports of surgical treatment of IAA
and APW with successful results have been reported in the
literature [6,7]. In our case, the complex lesions of the great
arteries was complicated by intracardiac finding of a CAVSD with
a single papillary muscle in the left ventricle and
intraoperative finding of a very poorly ventilated hypoplastic
left lung. Although a satisfactory repair of the arch and the
aortopulmonary window was obtained with a minimal gradient
across the aortic anastomosis of 8 mmHg, the intraoperative
decision to go for a staged approach rather than attempt to
correct the CAVSD in the same procedure was made [8].
As echocardiographic AV valve index was
borderline, our patient could not be clearly classified as
unbalanced or balanced [9]. The decision for a staged approach
was made based on the facts that there was a high probability of
an unbalanced left ventricle with paraschute deformity of the
left sided atriventricular valve and that a univentricular
repair would be a more probable option. The great concern was
the poorly developed and ventilated left lung, technical
challenges for the surgeon and associated anomalies (IAA, APW).
Although there are no established guidelines
in unbalanced CAVSD for deciding between biventricular or
univentricular repair, AV valve index could effectively
characterizes the anatomic substrate and selects surgical
strategy [9,10]. The main item of the operative strategy for
CAVSD, in setting of great arteries anomalies, is to classify
the CAVSD as balanced or unbalanced.
Contributors: All persons designated as
authors qualified for the authorship. They reached authorship
credit by contributions in concept, design and article drafting.
Also they helped with final approval of the version to be
published.
Funding: None; Competing
interests: None stated.
References
1. Browdie DA, Norberg W, Devig P, Atwood
G, Damle J, Agnew R, et al. Surgical management in
interrupted aortic arch and atrioventricular canal. J Thorac
Cardiovasc Surg. 1984;88:764-9.
2. McElhinney DB, Paridon S, Spray TL.
Aortopulmonary window associated with complete
atrioventricular septal defect. J Thorac Cardiovasc Surg.
2000;119:1284-5.
3. Brown JW, Ruzmetov M, Okada Y, Vijay
P, Rodefeld MD, Turrentine MW. Outcomes in patients with
interrupted aortic arch and associated anomalies: a 20-year
experience. Eur J Cardiothorac Surg. 2006;29:666-74.
4. Oosterhof T, Azakie A, Freedom RM,
Williams GW, McCrindle WB. Associated factors and trends in
outcomes of interrupted aortic arch. Ann Thorac Surg.
2004;78:1696-702.
5. Backer CL, Mavroudis C. Surgical
management of aortopulmonary window: a 40-year experience.
Eur J Cardiothorac Surg. 2002;21:773–9.
6. Davies MJ, Dyamenahalli U, Leanage RR,
Firmin RK. Total one-stage repair of aortopulmonary window
and interrupted aortic arch in a neonate. Pediatr Cardiol.
1996;17:122-4.
7. Konstantinov IE, Karamlou T, Williams
WG, Quaegebeur JM, DeNido PJ, Spray TL, et al.
Surgical management of aortopulmonary window associated with
interrupted aortic arch: A Congenital Heart Surgeons Society
study. J Thorac Cardiovasc Surg. 2006;131:1136-41.
8. Codispoti M, Mankad PS. One-stage
repair of interrupted aortic arch, aortopulmonary window,
and anomalous origin of right pulmonary artery with
autologous tissues. Ann Thorac Surg. 1998; 66:264-7.
9. Jegatheeswaran A, Pizarro C, Caldarone
CA, Cohen MS, Baffa JM, Gremmels DB, et al.
Echocardiographic definition and surgical decision-making in
unbalanced atrioventricular septal defect. Circulation.
2010;122: S209-15.
10. Kim WH, Lee TY, Kim SC, Kim SJ, Lee YT. Unbalanced
atrioventricular septal defect with parachute valve. Ann Thorac
Surg. 2000;70:1711-2.
|

