|
|
|
Indian Pediatr 2009;46: 127-132 |
 |
Neonatal Hypoglycemic Brain Injury - A Common
Cause of Infantile-onset Remote Symptomatic Epilepsy |
|
V Udani, P Munot, M Ursekar and S Gupta
From PD Hinduja National Hospital and Medical Research
Center; and Jhankaria Imaging Center, Mumbai, India.
Correspondence to: Vrajesh Udani, Child Neurology and
Epilepsy, PD Hinduja National Hospital and MRC,
Veer Savarkar Marg, Mahim, Mumbai 400 016, India.
Manuscript received: February 27, 2006;
Initial review completed: June 5, 2006;
Revision accepted: May 1, 2008. |
|
Abstract
Objectives:
To study the etiology of remote symptomatic epilepsy with onset in the
first 3 years of life. Patients with neonatal hypoglycemic brain injury
(NHBI), were further studied for risk factors and clinical features.
Methods: The study was conducted at a tertiary
pediatric neurology service between May-August 2004. Consecutive
patients were recruited prospectively. The probable etiological
diagnoses were based primarily on cranial imaging. Two radiologists,
blinded to the etiological diagnosis, reviewed the cranial imaging and
suggested the likely etiology based on published imaging criteria. There
were three categories i.e, (i) perinatal encephaloclastic
conditions (PEC) e.g., hypoxic ischemic encephalopathy (HIE) etc, (ii)
developmental (DV) e.g., tuberous sclerosis, etc and (iii)
postnatal (PN) e.g., trauma, etc. Three risk factors (birth weight, type
of delivery, feeding difficulty) were compared between NHBI and
developmental etiology (DV) groups. Neurological findings were compared
between the NHBI vs the other perinatal groups. Seizure details
were studied only in the NHBI group.
Results: 63 boys and 37 girls were recruited.
Mean age of seizure onset was 13.9 months. PEC were seen in 50 patients,
DV in 28 patients and PN in 5. NHBI was seen in 23 patients and was the
most frequent cause of epilepsy. Low birth weight (LBW), neonatal
feeding difficulties and cesarean delivery were significant risk factors
for NHBI vis-à-vis the DV group. Microcephaly, autism, visual
impairment and apraxia of hand use were common while spasticity or
dystronia were rare in NHBI. Spasms were the commonest seizure type.
Conclusion: Neonatal hypoglycemia is the most
common etiology of remote symptomatic infantile onset epilepsy. LBW,
poor neonatal feeding and cesarean delivery are significant clinical
correlates.
Keywords: Epilepsy, Etiology, Hypoglycemia, Infant, Seizure.
|
|
Epilepsy has its
highest incidence in infancy(1). At this time a unique interface exists
between normal brain maturation and the epilepsy, which may have profound
effects on the infant’s cognitive development. The etiology of infantile
remote symptomatic epilepsy is different from those at other ages. In
developed countries these appear to be mainly developmental disturbances
of cortical architecture i.e., cortical dysplasias (CDs),
agyria-pachygyria complex, tuberous sclerosis (TS) etc.(2,3).
Experience from developing nations(4-9) and past studies from many
developed nations(10) implicate perinatal encephaloclastic (PE)
(brain-damaging) conditions as major contributors for remote symptomatic
epilepsy, especially for West syndrome. Neonatal hypoglycemic brain injury
(NHBI) seemed to be an important risk factor in the 1960s in Finland;
however it ceased to be a risk factor in a subsequent study by the same
authors(10).
Initially we determined the probable etiology of remote
symptomatic epilepsy with onset within the first three years of life. We
then studied the patients with probable NHBI in greater detail as an
extension of the first study as this was found to be the single most
frequent cause.
Methods
The study was conducted in the child neurology section
at a tertiary care outpatient service in a large metropolitan Indian city
between May-August 2004. Consecutive patients were prospectively recruited
if they had onset of remote symptomatic epilepsy (RSE) in the first three
years of life. Only those with imaging documented lesions or confirmed
genetic/metabolic disorders underlying their epilepsy were included.
Children with acute symptomatic seizures, patients without available
imaging and where the age of seizure onset was not clear were excluded.
Seizure details, developmental milestones, and response to therapy were
obtained from the primary caregiver and supplemented with available
records. Gestational age, birthweight, presence of encephalopathy (defined
as a combination of seizures, altered sensorium) and the day it occurred,
feeding difficulties, mode of delivery and details of laboratory
investigations were specifically noted in the perinatal history.
Two radiologists, blinded to the clinical history,
reviewed the cranial MRIs or CTs and suggested a probable etiological
diagnosis, using standard imaging criteria. Whenever required new imaging
(usually MRI) was performed. The criteria used were previously published
criteria for diagnosis of PEC i.e. NHBI(11,12), periventricular
leucomalacia (PVL)(13) HIE(14), focal infarcts(FI) and others e,g.,
disproportionate involvement of parietal and occipital cortices and
sub-cortical white matter lesions are the hallmark of neonatal
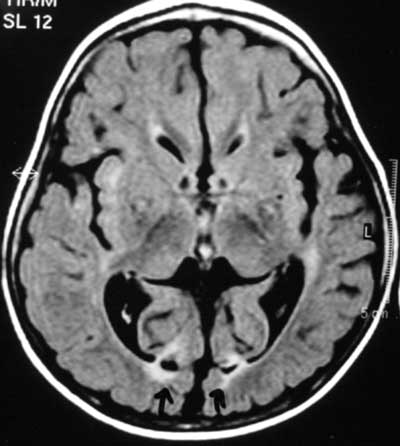
hypoglycemia(11,12) (Fig.1) while white matter
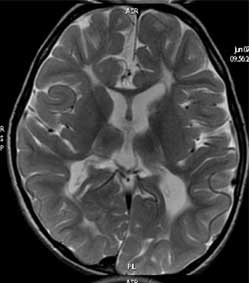
hyperintensity with or without loss of white matter and ventriculomegaly
is typical of PVL (Fig. 2)(13). The kappa coefficient was
determined to quantify the degree of inter-observer variation.
 |
|
Fig. 1 Bilateral occipital lesions
typical of neonatal hypoglycemic brain injury (MR FLAIR image). |
 |
|
Fig.2 Periventricular leukomalacia
hyperintensities in white matter, loss of white matter and
ventriculomegaly (T2 MR image). |
The study patients were ascribed a ‘probable
etiological diagnosis’ based primarily on the imaging findings. The three
etiological categories were (1) Perinatal enceplialoclastic conditions
(PEC) e.g., HIE, NHBI, PVL, FI; (2) developmental (DV) e.g.,
tuberous sclerosis (TS), cortical dysplasia (CD), metabolic, and (3)
postnatal (PN) e.g., trauma, meningitis. The diagnosis was regarded
as confirmed only if both radiologists concurred. In case of disagreement
between the two observers, laboratory tests and / or a suggestive history
were used to finally include or exclude the patient from a particular
diagnostic category. A few patients had unequivocal diagnoses not based on
imaging but on past clinical or non-imaging methods. In those where the
radiologists disagreed and there was no supportive evidence to back any
diagnosis, the case was classified as undefined.
Neonatal hypoglycemic brain injury (NHBI) was found to
be the single most frequent risk factor in the study and hence was studied
further. In the NHBI group the diagnosis (primarily based on MRI) was
correlated with the neonatal clinical history and blood glucose levels (if
available). We ascertained the frequency of three risk factors
(birthweight, mode of delivery and perinatal feeding difficulties) in the
NHBI group. These three were chosen as the caregivers reliably remembered
these three factors even when birth records were unavailable (which is
often the case in our country). We used the DV group as controls and
compared the same three factors in the two groups using a univariate
analysis. We chose the DV group as controls as this group would be more
likely to have an uneventful perinatal period and resemble healthy
controls.
Neurological and developmental findings were described
and compared in the different perinatal groups by the chi-square test.
Types of seizures and response to treatment were studied however only in
the NHBI group.
Results
One hundred patients (63 M 37 F) were recruited over a
period of three months. Mean age of seizure onset was 13.9 months (1-36
months). In 88/100 patients, the diagnoses of the radiologists completely
concurred. The kappa coefficient for diagnosis of NHBI was 0.83, for HIE
0.79, for PVL 0.63 and for developmental anomalies, 1. The etiological
diagnosis was reached in only 83 study patients as in 5 patients the
imaging abnormalities were considered not specific by both radiologists.
PE etiologies were seen in 50 patients (NHBI 23, HIE 8, PVL 7, focal
infarcts 9, multiple etiology 3), DV in 28 (tuberous sclerosis and
migration defect 9 each; Aicardi syndrome 4; metabolic 3 and others 3) and
post-natal in 5 (post encephalitic 2, head injury, neurocysticercosis and
medial temporal sclerosis in 1 each). In 17 the diagnosis remained
undefined.
In the NHBI group, 14/23 children had documented low
blood glucose in the neonatal period; the remaining 9 did not have any
birth records available though all had a compatible perinatal history with
encephalopathy between day two and four. Conversely, 9 patients with
documented low blood glucose in the neonatal period did not have the
characteristic imaging findings of NHBI and were thus not included in this
group as our diagnoses were based primarily on imaging criteria.
Low birthweight (<2.5 kg) (LBW), history of poor
feeding in the newborn period and lower segment cesarean section (LSCS)
delivery were all found to be significant risk factors for NHBI on
univariate analysis (Table I). Surprisingly, 6/19 patients
where birth weights were available were >2.5 Kg, with 3 having a weight of
>3 kg.
TABLE I
Risk Factors for Neonatal Hypoglycemic Brain Injury (NHBI)
| Risk Factors |
NHBI
(Cases)
N= 23 |
Controls
N=28 |
P value |
| Birthweight |
|
|
|
| <2.5 kg |
13 |
3 |
<0.001 |
| 2.5-3 kg |
3 |
9 |
|
| >3 kg |
3 |
12 |
|
| Unknown |
4 |
4 |
|
| Poor feeding |
19 |
5 |
<0.001 |
| Cesarean delivery |
11 |
6 |
0.046 |
Table II lists and compares the
neurological findings in patients with NHBI with patients from other PEC
e.g. HIE, PVL etc. The clinical discriminatory features that
seemed to separate the NHBI group from other perinatal etiologies were the
relative lack of spasticity/ dystonia in these patients. Other features
frequently observed in children with NHBI in our study were microcephaly
(100%), autism (57%), apraxia of hand use (65%) and cortical visual
impairment (48%). Infantile spasms were the most common seizure type in
children with NHBI (n=12, 52%) followed by partial (22%),
generalized (17%) and mixed (9%) seizures. More than half of the patients
had refractory seizures.
TABLE II
Neurological Findings in Children with Pre/perinatal Encephaloclastic Etiology for Epilepsy
|
Neurological finding |
NHBI |
Other
perinatal etiology |
P value |
|
|
N=23 |
PVL |
HIE |
FI |
Total |
|
| |
|
Dystonia |
2 |
1 |
5 |
2 |
8 |
0.09 |
|
Spasticity |
4 |
2 |
5 |
9 |
16 |
<0.001 |
|
Autism |
13 |
4 |
7 |
1 |
12 |
NS |
|
Severe MR |
11 |
3 |
7 |
1 |
11 |
NS |
|
Visual impairment |
11 |
3 |
7 |
3 |
13 |
0.07 |
NHBI: neonatal hypoglycemic brain injury, PVL: periventricular leucomalacia,
HIE: hypoxic-ischemic encephalopathy,
FI: focal infarcts, NS: not significant.
|
Discussion
We used neuroimaging as the primary method for
establishing the etiology of the epilepsy as clinical histories / hospital
birth records are often unavailable or incomplete in our country. Ideal
methods to establish an etiology would be to rely on clinical, laboratory
and imaging findings in cohorts who are either prospectively followed up
from the newborn period or looking at carefully documented antece-dent of
patients with early onset epilepsy. In India, these two approaches are
rarely possible in the general population. Often clinical details of the
perinatal period are sketchy and laboratory investigations are either not
performed or poorly documented. The parents are often unaware of the
details or have forgotten them. Moreover, prospectively followed up
cohorts from tertiary care centers (where neonatal care is of a high
standard and homogeneous) may not reflect the reality in general
populations where perinatal care is much more heterogeneous. The
reliability of neuroimaging in diagnosing etiologies in epilepsy syndromes
is well established(11-14). The excellent inter-observer agreement for
imaging findings noted in our study further reinforces the accuracy of the
study results.
Our findings on causes of remote symptomatic epilepsy
have been reported earlier in retrospective series from developing
nations(4-9) and from older studies in Finland(10). However our results
are in contrast with the etiology of infantile remote symptomatic epilepsy
from developed nations where progressive encephalopathies and
developmental disturbances of cortical architecture (cortical dysplasias,
neuronal migration disorders, tuberous sclerosis) are the main
causes(2,3). Perinatal care in developing countries is often rudimentary
in many primary centers and is the probable explanation for this
difference. NHBI has been almost eliminated from Western world nurseries
as blood glucose is routinely monitored in high risk groups including LBW
infants. It remains an important cause of epilepsy in developing countries
like Argentina(15), where 13/15 patients had typical parietooccipital
lesions as in our study and many had mental retardation and visual
impairment on followup. It is possible that the contribution of NHBI was
underestimated in our study, as 9 children with documented neonatal
hypoglycemia were not included because their MRIs were not characteristic.
An interesting observation in our study is the
occurrence of NHBI even in appropriate for gestational age (AGA) newborns.
Maternal diabetes may have been a risk factor though details are
unavailable in our cohort. Late establishment of feeding even in AGA
babies may also predispose to hypoglycemia and NHBI.
LSCS rates were significantly higher in the NHBI group
vis-à-vis the DV group. We took the latter as controls as this
group presumably had similar risk factors as healthy newborns. Higher LSCS
rates could be partly explained on the general higher rates in LBW
infants(16) as well as the general increase in LSCS rates in India(17). An
LSCS delivery is often responsible for delayed establishment of
breastfeeding(18) (due to maternal sedation / pain) and the risk of
hypoglycemia is increased in LBW babies. Our study reinforces the need to
maintain vigilance for hypoglycemia in all babies delivered by LSCS,
especially those below 3 kg.
Children with NHBI had lower rates of dystonia and
spasticity. Epilepsy, mental retardation, micro-cephaly and visual
disturbances were seen often and have been reported following NHBI(19).
However, the high frequency of autism and apraxia of hand use, which was
seen in more than half our patients, has not been highlighted in the
literature. Autism may be related to uncontrolled infantile spasms(20) and
apraxia of hand use may be because of the frequent damage to the parietal
association areas in NHBI. The lack of damage to motor pathways including
the basal ganglia probably explains the low frequency of tone
abnormalities in NHBI. The absence of significant motor changes
discriminates these infants from survivors of HIE, PVL and stroke.
The seizure types commonly encountered were infantile
spasms and partial seizures, which are similar to other series(21). What
was alarming was the high incidence of refractory epilepsy, though there
were others who had only infrequent epilepsy with near normal development
suggesting that there is a wide spectrum of disabilities. This is also
highlighted in the Argentinian study where many patients had a fairly good
outcome(15). Micro-cephaly was seen even in those mildly affected, as in
our group.
These results from a child neurology center would be
subject to a selection bias and therefore certain etiologic groups may
have been over-represented. However, it emphatically establishes the
contribution of perinatal insults, particularly neonatal hypoglycemia as
an important cause of the childhood epileptic burden and invokes a need to
improve perinatal care in our country.
Contributors: VU: Concept and design, analysis and
interpretation of data, revising the draft critically for important
intellectual content; and final approval of the version to be published;
PM: data acquisition and initial draft of manuscript; MU: analysis and
interpretation of data; revising the draft critically for important
intellectual content; and SG: analysis and interpretation of data;
revising the draft critically for important intellectual content.
Funding: None.
Competing interests: None stated.
|
What is Already Known?
• In developed nations, the
etiology of epilepsy with onset in the first 2-3 years of life is
usually due to prenatal etiologies.
What This Study Adds?
• Perinatal brain-damaging etiologies, especially
neonatal hypoglycemia are responsible for half the symptomatic
epilepsies in the first 3 years of life. |
References
1. Freitag CM, May TW, Pfafflin M, Konig S, Rating D.
Incidence of epilepsies and epileptic syndromes in children and
adolescents: a population-based prospective study in Germany. Epilepsia
2001; 42 : 979-985.
2. Nelson KB, Ellenberg JH. Predisposing and causative
factors in childhood epilepsy. Epilepsia 1987; 28 Suppl 1: S16-24.
3. Rantala H, Ingalsuo H. Occurrence and outcome of
epilepsy in children younger than two years. J Pediatr 1999; 135: 761-764.
4. Aydinli N, Caliskan M, Ozmen M, Tonguc E.
Neuroradiologic aspects of West syndrome. Pediatr Neurol 1998; 19 :
211-216.
5. Singhi P, Ray M. Profile of West syndrome in North
Indian children. Brain Dev 2005; 27:135-140.
6. Kalra V, Gulati S, Pandey RM, Menon S. West syndrome
and other infantile epileptic encephalopathies–Indian hospital experience.
Brain Dev 2002; 24: 130-139.
7. Salonga AM, Lukban MB, Ortiz MH, Balatero-Terencio
B, Lagman AM. West syndrome: the Philippine experience. Brain Dev 2001;
23: 616-623.
8. Kwong KL, Chak WK, Wong SN, So KT. Epidemiology of
childhood epilepsy in a cohort of 309 Chinese children. Pediatr Neurol
2001; 24: 276-282.
9. Chawla S, Aneja S, Kashyap R, Mallika V. Etiology
and clinical predictors of intractable epilepsy. Pediatr Neurol 2002; 27:
186-191.
10. Riikonen R. Decreasing perinatal mortality:
unchanged infantile spasm morbidity. Dev Med Child Neurol 1995; 37:
232-238.
11. Barkowich AJ, Ali FA, Rowley HA, Bass N. Imaging
patterns of neonatal hypoglycemia. AJNR 1998; 19: 523-528.
12. Spar JA, Lewine JD, Orrison WW. Neonatal
hypoglycemia: CT and MR findings. AJNR 1994; 15: 1477-1478.
13. Baker LL, Stevenson DK, Enzmann DR. End-stage
periventricular leukomalacia: MR evaluation. Radiology 1988; 168 :
809-815.
14. Gururaj A, Sztriha L, Dawodu A, Nath KR, Varady E,
Nork M, et al. CT and MRI patterns of hypoxic-ischemic brain damage
following perinatal asphyxia. J Trop Pediatr 2002; 48: 5-9.
15. Caraballo RH, Sakr D, Mozzi M, Guerrero A, Adi JN,
Cersosimo RO, et al. Symptomatic occipital lobe epilepsy following
neonatal hypoglycemia. Pediatr Neurol 2004; 31: 24-29.
16. Smith GC. A population study of birth weight and
the risk of caesarean section: Scotland 1980-1996. BJOG 2000; 107:
740-744.
17. Pai M, Sundaram P, Radhakrishnan KK, Thomas K,
Muliyil JP. A high rate of caesarean sections in an affluent section of
Chennai: is it cause for concern? Natl Med J India 1999; 12: 156-158.
18. Chye JK, Zain Z, Lim WL, Lim CT. Breast feeding at
6 weeks and predictive factors. J Trop Pediatr 1997; 43: 287-292.
19. Menni F, de Lonlay P, Sevin C, Touati G, Peigne C,
Barbier V, et al. Neurologic outcomes of 90 neonates and infants
with persistent hyper-insulinemic hypoglycemia. Pediatrics 2001; 107:
476-479.
20. Askalan R, Mackay M, Brian J, Otsubo H, McDermott
C, Bryson S, et al. Prospective preliminary analysis of the
development of autism and epilepsy in children with infantile spasms. J
Child Neurol 2003; 18 :165-170.
21. Hamer HM, Wyllie E, Luders HO, Kotagal P, Acharya
J. Symptomatology of epileptic seizures in the first three years of life.
Epilepsia 1999; 40 : 837-884. |
|
|
 |
|

