Sandip Bartakke, S.B. Bavdekar, Pratima Kondurkar,
Mamta N. Muranjan, Mamata V. Manglani* and Ratna Sharma*
From the Department of Pediatrics, Seth G.S. Medical
College and KEM Hospital and *Division of Pediatric
Hematology-Oncology, L.T.M. Medical College and L.T.M. General
Hospital.
Mumbai, India.
Correspondence to: Dr. S.B. Bavdekar, A2-9, Worli
Seaside CHS, Pujari Nagar, KAG Khan Road, Worli, Mumbai 400 018,
India. E-mail:
[email protected]
Manuscript received: June 24, 2003, Initial review
completed: October 27, 2003;
Revision accepted: September 6, 2004.
.
Abstract:
A prospective multi-centric study was
conducted to determine if iron-chelating agent deferiprone also
chelates zinc. Twenty four-hour urinary zinc levels were
compared in multiply transfused children with thalassemia major
not receiving any chelation therapy (Group A, n = 28), those
receiving deferiprone (Group B, n = 30) and age and sex-matched
controls of subjects in Group B (Group C, n = 29) by a
colorimetric method. The 24-hour mean urinary excretion of zinc
was significantly higher in Group B (1010.8 ± 1248.1 mcg) than
in the other two groups (Group A: 547.8 ± 832.9mcg and Group C
459.8 ± 270.3mcg) indicating that deferiprone chelates zinc.
Keywords: Adverse event, Chelation, Hemosiderosis, Iron.
Beta thalassemia is one of the most common
genetic defects seen in certain populations(1). Thalassemia major
represents the life threatening variety of this disorder wherein
the child’s life depends upon receipt of regular red cell
transfusions. Chronic iron overload leading to organ dysfunction
involving the heart, liver and endocrine glands is the logical
consequence of life-long transfusion therapy(2).
Chelation therapy with desferrioxamine (deferoxamine)
helps avert iron overload(3). However, the drug has to be
administered as a subcutaneous infusion 5-6 days a week. Cost of
therapy and need to administer it as an injection make many
families reluctant in initiating chelation therapy. Deferiprone
(L1 or 1, 2-dimethyl-3-hydxoxypyridin-4-one), the only oral
iron-chelating drug cleared for clinical use, is primarily being
prescribed in the developing countries. Although the drug
deferiprone offers an affordable option to children with
thalassemia major in developing countries, it is imperative that
investigators continue to study the drug. Gastrointestinal
disturbances, arthralgia, agranulocytosis and raised levels of
transaminases in the liver are some of its known side effects(4).
Being a chelator of iron, it is possible that the drug may also
chelate other metallic ions. Zinc is one of the important elements
that play an extensive role in various body functions. However,
limited information is available regarding the effect of
deferiprone adminis-tration on zinc status. Hence, the present
study was undertaken to determine the effect of deferiprone
therapy on urinary excretion of zinc.
Subjects and Methods
This prospective study was conducted at two
large tertiary care hospitals in Mumbai over a period of one year.
The institutional ethics committee endorsed the study protocol.
Multiply transfused children diagnosed to have thalassemia major
on the basis of clinical presentation, dependence on blood
transfusion and hemoglobin electrophoresis report were enrolled in
the study after obtaining an informed consent from the guardians.
Those not receiving any chelation therapy were included in Group A
while those receiving deferiprone were enrolled in Group B. In
addition, the age-and sex-matched children without thalassemia
major and not receiving any transfusion therapy were included in
the study as controls (Group C) after following a similar
procedure for obtaining the consent from the guardians. The
controls were also matched for the nutritional status. The data
regarding age at diagnosis, duration of transfusion therapy,
yearly trans-fusion requirements (transfusion required in the last
12 months), results of the liver function tests (plasma levels of
hepatic transaminases and protein), serum ferritin levels and the
dose and duration of deferiprone therapy was collected by review
of records. The nutritional status was deter-mined and the grade
of malnutrition was judged using the classification provided by
the Indian Academy of Pediatrics(5).
Two mL of venous blood was collected from
subjects using heparin as anticoagulant. The sample was
centrifuged and plasma was separated. Twenty-four hour, urine was
collected by patients at home in a zinc-free container. The volume
of the urine was measured in the laboratory. Samples of plasma and
urine were stored in the refrigerator at 2şc to 8şC and
determination of zinc levels in these samples was done in batches
of five samples. The laboratory technician who performed the assay
was unaware of the groups to which the subjects belonged. Zinc
level was estimated using a colorimetric method(6) using a kit
provided by Randox Laboratories. Comparison of a variable between
different groups was deter-mined using Student’s "t" test.
Correlation of different variables in a single group was done by
using coefficient of correlation.
Results
Eighty seven subjects (55 boys and 32 girls, M
: F = 1.6) were enrolled in the study that included 28 children in
Group A, 30 in Group B and 29 in Group C. As shown in Table I, the
three groups were comparable in terms of age and state of
malnutrition. Fifteen (50%) children in Group B were on
deferiprone for only 3-6 months, six were receiving the drug for
7-12 months while another 9 (30%) were on chelation therapy for
over 12 months. As shown in Table 1, the 24-hour urinary excretion
of zinc was significantly higher in children receiving deferiprone
(Group B, 1010.8 ± 1248.1 mcg) than in the other two groups.
Children with thalassemia major who were receiving regular
transfusion therapy, but were not receiving any chelation therapy
(Group A) did not show significantly high urinary zinc excretion
(547.8 ± 832.9 mcg/24 hr) as compared to the controls (Group C,
459.8 ± 270.3; P >0.05).
TABLE I
Demographic Characteristics, 24-hour Urinary Zinc Excretion and Plasma Zinc Levels.
|
Characteristics |
Group A |
Group B |
Group C |
Children with thalassemia major on
Regular transfusion therapy |
Controls |
Without chelation
therapy |
On deferiprone
|
|
Age (Mean ± SD; years) |
7.2 ± 2.5* |
7.9 ±3.4* |
7.2 ±2.9* |
|
Male: female ratio |
1.3:1 |
2: 1 |
1.9 : 1 |
|
Median grade of malnutrition |
II |
II |
II |
|
24-hr urinary zinc excretion (µg/24
hrs) |
547.8 ±832.9** |
1010.8 ±1248.1**# |
459.8 ±270.3# |
|
Plasma zinc level (µmol/L) |
8.0 ±5.8 |
7.5 ±6.0 |
9.5 ±3.8 |
*P > 0.05, ** P< 0.01; #: P<0.001.
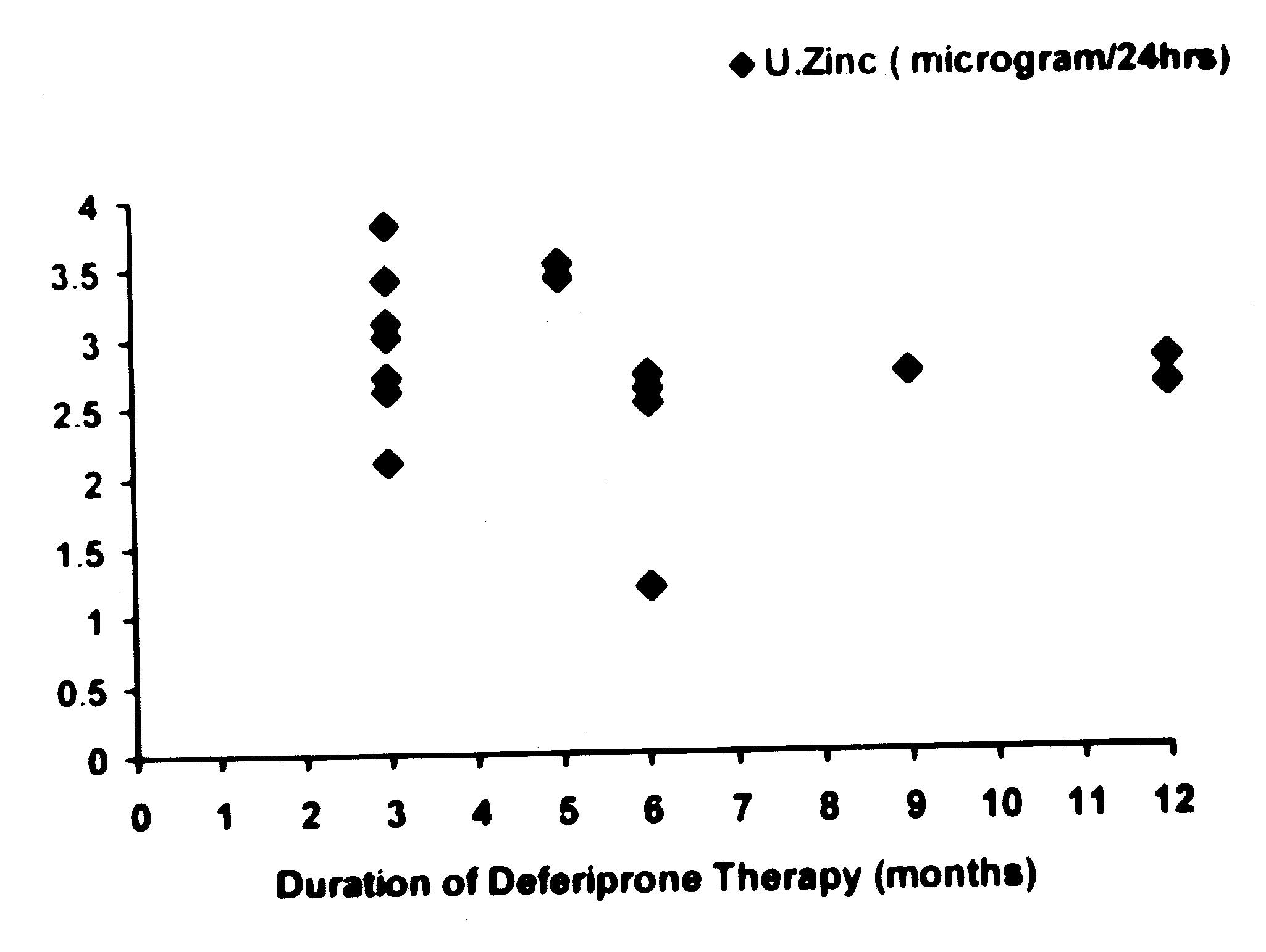
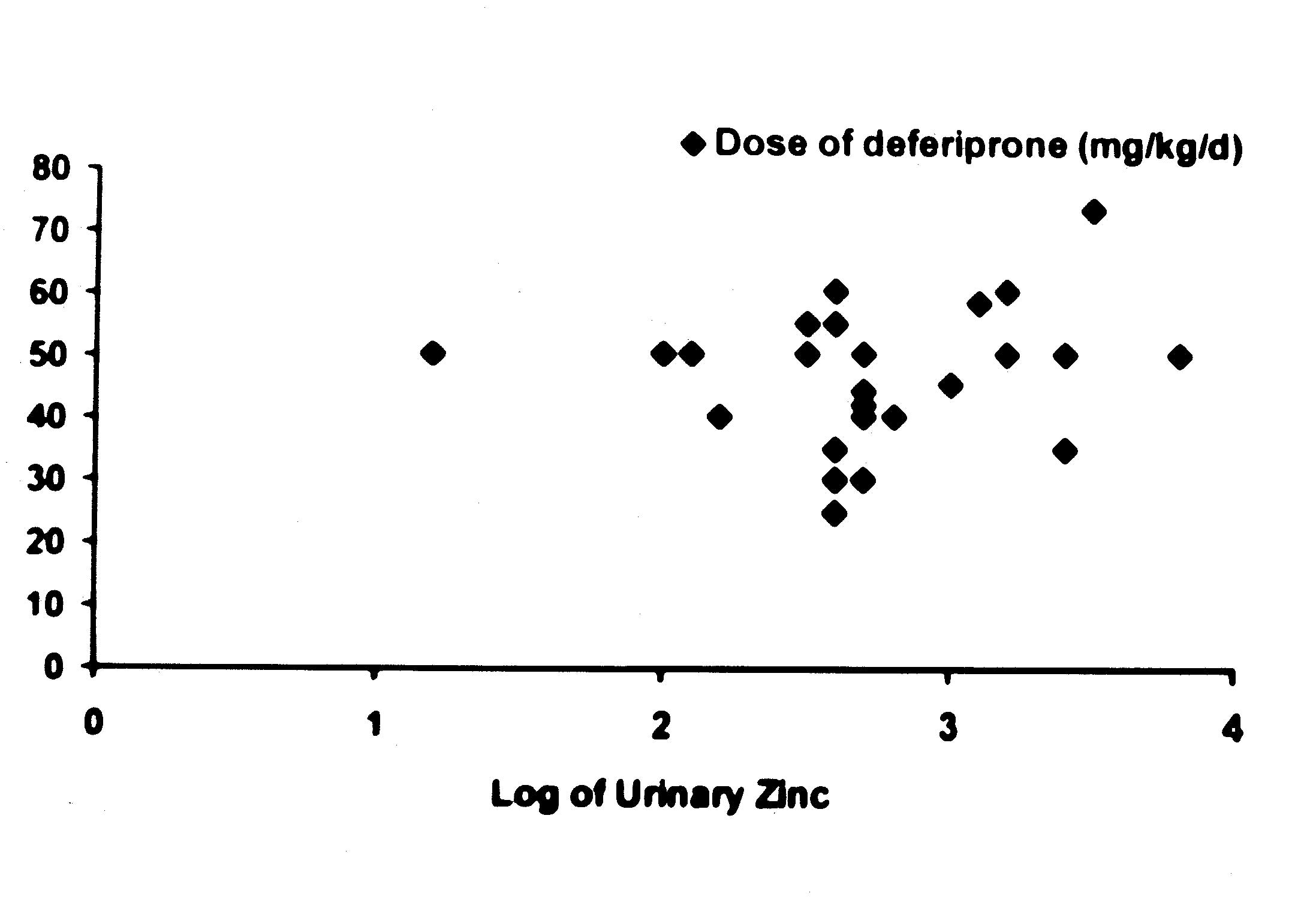
An attempt was made to determine if the urinary
excretion of zinc was related to the duration and dose of
deferiprone in the patients belonging to the Group B (Figs. l and
2). As the range of values for 24-hour urinary zinc excretion were
too wide, log of these values were compared with the duration and
dose of deferiprone therapy. It was noted that there was no
correlation between duration (r = – 0.26, P <0.05) and dose (r =
0.19, P <0.05) of deferiprone and (log of) urinary zinc levels.
Although plasma zinc levels in Group B were found to be lower than
those in other groups, the difference was not statistically
significant (P >0.05). However, it is noteworthy that the levels
in all the three groups were significantly lower than the
reference values(6).
 |
 |
| Fig. 1.
Relationship between the duration of deferiprone therapy and
urinary zinc excretion. |
Fig. 2.
Relationship between the dose of deferiprone and urinary
zinc excretion.
|
Discussion
A few studies have indicated that deferiprone
may, in addition to iron, chelate zinc as well. This is an
important issue since zinc plays an important role in several
physiological functions; protein synthesis, gene expression,
immunity, wound healing amd maintenance of integrity of
intra-cellular organelles(7). In addition, it has been recently
noted that deferiprone therapy is associated with zinc depletion
in thymocytes(8).
The present study demonstrated that urinary
zinc levels in children on deferiprone were significantly higher
than those in controls as well as in children with thalas-semia
who were not receiving deferiprone. This unequivocally proves that
the drug chelates zinc and it is not the "thalassemic state" that
is responsible for excessive zinc excretion. The study did not
demonstrate any significant relationship between dose of
deferiprone and duration of chelation therapy and urinary zinc
excretion. Al Refaie, et al.(9) also did not find correlation
between the dose of deferiprone and the extent of urinary zinc
excretion. It is possible that long-term deferiprone therapy
depletes total body zinc and therefore, urinary zinc excretion may
not increase any further or may, in fact, show a decline from the
baseline when the body zinc stores have been significantly
depleted.
The failure of the present study to demonstrate
this relationship may be due to the fact that up to 50% of our
subjects in Group B were receiving deferiprone for a short period
of 3-6 months. This may also account for the inability of the
study to demonstrate significantly lower plasma levelsof zinc in
patients receiving deferiprone.
Despite certain limitations, the findings
obtained from the study have definite implications. Deferiprone
chelates zinc and hence, with long-term use is likely to result in
depletion of body zinc. Cohen, et al.(4) and Al-Refaie, et al.(9)
have already shown that deferi-prone therapy results in low zinc
levels in the blood. Therefore, it may be prudent to start zinc
supplementation to avoid the occurrence of negative zinc balance.
This is probably more relevant in Indian children as they are
known to have zinc levels that are lower than reference
values(10).
Acknowledgement
The authors thank Dr. Nilima Kshirsagar, Dean,
Seth G.S. Medical College and KEM Hospital, Mumbai for permitting
the authors to publish this study.
Contributors: SB data collection, statistical
analysis, providing first draft; SBB involved in
conceptualization, designing of the study, editing drafts, will
act as guarantor; PK performed technical and laboratory tests; MNM
supervision over data collection, edited drafts; MVM: assisted in
designing and coordinating the study, edited drafts; RS:
supervision over data collection, assisted in literature review,
edited drafts.
Funding: None.
Competing interests: None declared.
|
Key
Messages |
• Administration of deferiprone is associated with an increase
in the urinary excretion of zinc.
• Further research is warranted on the need to provide zinc
supplementation to children receiving deferiprone for a longer
duration.
|
|
|
1. Indian Council of Medical Research.
Multicentric study to determine b-thalassemia carrier rate
in school children in Bombay, Calcutta and Delhi, New Delhi,
Indian Council of Medical Research 1991.
2. Edward JB, Patricia JVG. Thalassemia
Syndromes. In: Miller DR, Robert LB (Eds.) Blood Diseases of
Infancy and Childhood, 7th edition, St. Louis; Mosby;
1990,460-498.
3. Olivieri NF, Brittenham GM.
Iron-chelating therapy and the treatment of thalassemia.
Blood 1997; 89: 739-761.
4. Cohen AR, Galanello R, Piga A, Dipalma
A, Vullo C, Tricta F. Safety profile of the oral chelator
deferiprone: A multi-centre study. Br J Hematol 2000; 108:
305-312.
5. Nutrition subcommittee of the Indian
Academy of Pediatrics. Report. Indian Pediatrics 1972, 9:
360.
6. Makino T, Saito M, Horiguchi D, Kina
K. A highly sensitive colorimetric determination of serum
zinc using water soluble pyridylazo dye. Clinical Chimica
Acta 1982; 120: 127- 135.
7. Hipolito VN, Ananda SP. Vitamins &
Trace elements In: Alex CS, Leonard J (Eds.) Gradwohl’s
Clinical Laboratory Methods and Diagnosis. 8th edition, St.
Louis; The C.V. Mosby Company. 1980; p. 376-378.
8. Maclean KH, Cleveland JL, Porter JB.
Cellular zinc content is a major determinant of iron
chelator-induced apoptosis of thymocytes. Blood 2001; 13:
3831-3839.
9. Al-Refaie FN, Wonke B, Hoffbrand AV,
Wickens DG, Nortey P, Kontoghiorghes GJ. Efficacy and
possible adverse effects of the oral iron chelator 1,2
dimethyl 3-hydroxypyrid- 4- one (Ll) in thalassemia major.
Blood 1992; 80: 593-599.
10. Lahiri K, Kher A, Rathi S. Zinc: In Health and
Disease. In: Gupte S. (Ed.) Recent Advances in pediatrics.
Special Volume Nutrition, Growth and Development. New Delhi:
Jaypee Brothers Medical Publishers (P) Ltd; 1997. p. 287-
298.
.
|
