|
|
|
Indian Pediatr 2020;57:
1172-1176 |
 |
What Is New in the Management of Childhood Tuberculosis
in 2020?
|
|
Varinder Singh 1 and Ankit
Parakh2
From 1Department of Pediatrics, Lady Hardinge Medical College and
Kalawati Saran Children’s Hospital; and 2Pediatric Pulmonology and Sleep
Medicine, BL Kapur Memorial Hospital; New Delhi, India.
Correspondence to: Dr Varinder Singh, Director-Professor, Department
of Pediatrics, Lady Hardinge Medical College and Kalawati Saran
Children’s Hospital, New Delhi 110 001, India.
Email: [email protected]
|
|
The Government of India has developed
a National Strategic Plan for tuberculosis (TB) elimination by 2025,
five years ahead of the global target set by the World Health
Organization (WHO). For achieving these targets there has been a
paradigm shift in the diagnostic and treatment strategies of TB at all
ages. This update summarizes the specific changes in pediatric TB
management in light of the guidelines developed by National Tuberculosis
Elimination Program and Indian of Academy of Pediatrics.
Keywords: Diagnosis, End TB
strategy, National strategic plan, National Tuberculosis Elimination
Program.
|
|
W orld Health Organization (WHO)
announced the End TB strategy with the target of reducing tuberculosis
(TB) deaths by 90% and 95%, and, incidence by 80% and 90%, by 2030 and
2035, respectively [1]. However, Government of India has decided to aim
TB elimination from our country by 2025, ahead of the global target [2].
Childhood TB is an important area of intervention while drawing the
road-map to end TB. National Tuberculosis Elimination Program (NTEP) and
Indian of Academy of Pediatrics (IAP) have partnered to develop updated
guidelines and training program for management of childhood TB in the
country. The salient updates are detailed below.
NEW PARADIGM OF TB DIAGNOSIS
There is a paradigm shift in diagnostic strategy from
conventional smear microscopy to molecular methods of diagnosis due to
their higher sensitivity. NTEP approved rapid nucleic acid amplification
tests (NAAT) like Xpert Rif/Truenat
have made it possible to detect Mycobac-terium
tuberculosis (MTb) with much higher sensitivity as compared to smear
and rapidity than culture. The testing turnaround time for rapid NAAT is
2 hours. These tests are also nested for establishing rifampicin
resistance - a surrogate for multi-drug resistant (MDR) TB.
In addition, NTEP also recommends Line probe assays
(LPA) – which are multiplex NAAT- to test for resistance to rifampicin,
isoniazid and other second line drugs (flouroquinolones and second line
injectables). Unlike rapid NAAT, LPA due to its relatively lower
sensitivity can be used directly only in smear positive specimens or
else after isolating MTb on culture, and has a turnaround time of 3-4
days.
So, TB diagnostics have now graduated to upfront
testing every likely patient for presence of MTb as well as rifampicin
resistance under the strategy called universal drug sensitivity testing
(U-DST) [3].
How Does It Impact the Diagnosis of TB in Children?
Conventional TB diagnostics for children involved
appropriate use of clinical details, chest radiology and tuberculin skin
test, with much less focus on micro-biology, due to poor yield (AFB
smear) or access issues (MTb cultures). U-DST strategy has led to change
in the diagnostic pathways to include NAAT for every patient where a
biological specimen can be procured. Routine chest imaging is done as
initial screening test as testing of respiratory specimens from
radiologically positive cases improves the yield of NAAT [4,5].
While NAAT has higher sensitivity than the smear, yet
it fails in many paucibacillary cases. It is good only as a ‘rule in’
test and a negative NAAT does not rule out TB. The conventional methods
of clinical diagnosis still need to be relied upon among those who are
not confirmed by molecular tests. Current algorithm for evaluation of a
child with pulmonary TB is shown in Fig. 1.
MANAGEMENT OF CHILDHOOD TB
What Is New in Treatment?
Treatment of TB has also evolved from erstwhile
standard regimens based on the likely risk of drug resistance (new
versus retreatment cases) to regimens based on identification of key
resistance. The evidence available earlier in 70s suggested that the
retreatment cases could be treated with a simpler 5 drug category II
regimen. However, post implementation operational research and
meta-analysis showed that this strategy was associated with increased
risk of treatment failure with amplification of resistance to other
companion drugs, particularly, if the patient was initially harboring
rifampicin resistance [3,6]. With the feasibility of upfront rapid
testing for rifampicin resistance, use of standard regimens without
sensitivity testing is no more recom-mended for both the new as well as
retreatment cases.
Likewise, the non-responders to initial regimen for
drug sensitive TB (by 4 weeks) should be assessed again for presence of
drug resistance (rifampicin and isoniazid at least). Non responsive
cases with resistance to rifampicin and/or isoniazid are also tested for
resistance to second line drugs like fluoroquinolones and the injectable
aminoglycosides to provide the most suited regimens depending on the
resistance pattern. The effort is to manage the cases as per the
sensitivity to key drugs, thus improving outcomes and preventing further
ampli-fication of drug resistance. Retreatment cases with-out rifampicin
resistance are now treated again with initial 4 drug regime while being
tested for isoniazid resistance. In case of isoniazid (mono- or poly-)
resistance, 6 month uniphasic 4 drug regime, where isoniazid is replaced
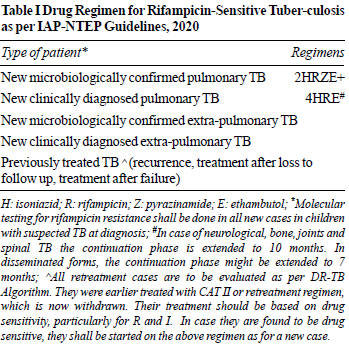
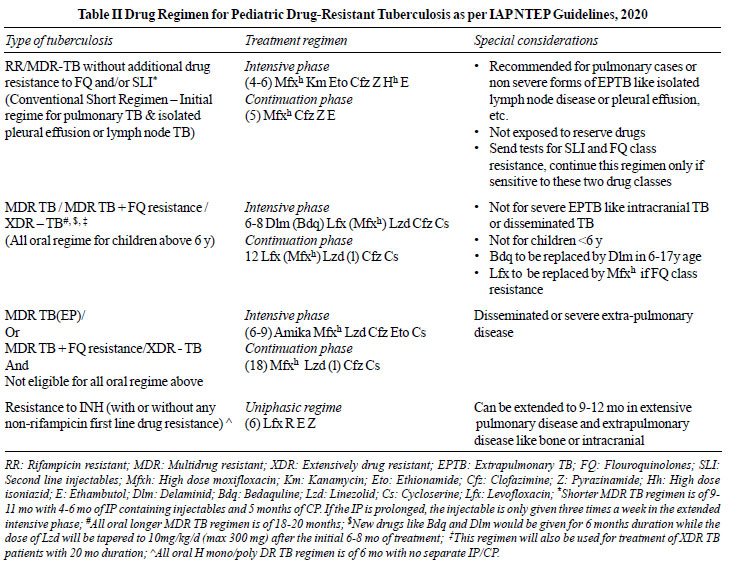
by levofloxacin, is recommended. The IAP NTEP 2020 TB treatment
guidelines are shown in Table I and II. The
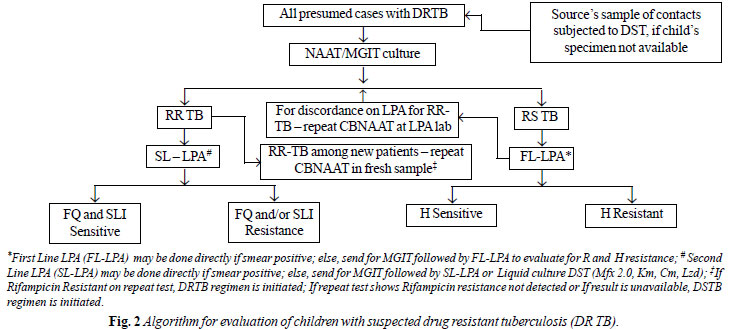
algorithm for evaluation of children with suspected drug resistance is
shown in Fig. 2.
 |
| |
| |
 |
| |
 |
| |
| |
 |
What Else Is New in Management?
NTEP has introduced daily therapy with dispersible
tablets in fixed dose combinations (FDC) for children. Younger children
get 3-drug FDCs (HRZ) along with 100 mg ethambutol tablets. Older
children can also get, in addition, 4-drug FDCs (RHZE) to meet their
drug dosages (Table III). Isoniazid and rifampicin are in
a ratio of 2:3 and the average dose of isoniazid is around 10 mg/kg/day.
These drugs are given free of cost in public sector and the private
sector can also access these drugs for free through various partnership
schemes that the program offers.
Table III Tuberculosis Drug Formulations and Dosages for Children As per IAP NTEP Guidelines, 2020
| Weight band (kg) |
Dose from 0-18 y* |
| 4-7 |
1P + 1E |
| 8-11 |
2P + 2E |
| 12-15 |
3P + 3E |
| 16-24 |
4P + 4E |
| 25-29 |
3P+3E+1A |
| 30-39 |
2P+2E+2A |
|
H-Isoniazid, R-Rifampicin; Z-Pyrazinamide, E-Ethambutol;
*number preceding the letter denotes number of pediatric or
adult formulations; IAP: Indian Academy of Pediatrics; NTEP:
National Tuberculosis Elimination Program; Pediatric formulation
(P) H50, R75, Z150 + E 100 (E separate tab); adult formulation
(A) H75, R150, Z400, E275; Children (aged 0-18 y)
upto the weight of 39 kg should be managed as per this table;
children (aged 0-18 y) ³40 kg would be managed as per the
various weight bands described for adults. |
Experts now also recommend addition of pyridoxine (10
mg/day) with isoniazid containing regimens because of the risk of
peripheral neuropathy due to higher dosages of isoniazid and high
prevalence of malnutrition amongst the affected.
Current Status of Preventive Treatment
Goal to eliminate TB cannot be achieved timely unless
the pool of cases with latent infection is treated. TB preventive
treatment may now be extended to all household contacts of an infectious
case after ruling out disease by symptom screening in line with WHO
guidance. For children above 5 years, if facilities exist, one may test
for presence of latent infection and then treat. But it is not mandatory
to test for infection due to lack of simple and affordable point of care
tests. TB preventive treatment is also recommended for any tuberculin
skin test positive child who is receiving immunosuppressive therapy
(children with nephrotic syndrome, acute leukemia, etc.), and a
child born to mother who was diagnosed to have TB in pregnancy but has
no evidence of disease [7].
Isoniazid is recommended for TB preventive treatment
at a dose of 10 mg/kg/day for six months. No drugs are currently
recommended for TB preventive therapy for the contacts of MDR TB cases
but a close follow up for two years after exposure is recommended for
timely identification of those developing disease among exposed [8].
To conclude, the management of TB in children now has
undergone a sea change with drug sensitivity directed therapy becoming
the corner pillar. NTEP approved rapid NAAT has become the core
investigative modality and erstwhile clinic-radiological approach of
diagnosis is used only when NAAT fails in a clinically probable case.
The pediatricians need also to be aware of the updated guidelines
detailing change in regimens and drug dosages so that they can
rationally manage TB among children.
Contributors: Both authors contributed to
reviewing the literature, drafting, correcting and finalizing the
manuscript.
Funding: None; Competing interest: None
stated.
REFERENCES
1. World Health Organization. The End TB Strategy.
WHO; 2015.
2. Ministry of Health with Family Welfare. National
Strategic Plan for Tuberculosis: 2017-25. Revised National Tuberculosis
Control Programme. March 2017. Accessed September 16, 2020. Available
from: https://tbcindia. gov.in/WriteReadData/National%20Strategic
%20Plan% 202017-25.pdf
3. Central TB Division, Ministry of Health with
Family Welfare. Technical and Operational Guidelines for TB Control in
India 2016. Revised National Tuberculosis Control Program. MoHFW.
Accessed September 16, 2020. Available from: www.tbcindia.gov.in
4. Raizada N, Sachdeva KS, Nair SA, et al.
Enhancing TB case detection: Experience in offering upfront Xpert
MTB/RIF testing to pediatric presumptive TB and DR TB cases for early
rapid diagnosis of drug sensitive and drug resistant TB. PLoS One.
2014;9:e105346.
5. Singh S, Singh A, Prajapati S, et al; Delhi
Pediatric TB Study Group. Xpert MTB/RIF assay can be used on archived
gastric aspirate and induced sputum samples for sensitive diagnosis of
paediatric tuberculosis. BMC Microbiol. 2015;15:191.
6. Menzies D, Benedetti A, Paydar A, et al.
Standardized treatment of active tuberculosis in patients with previous
treatment and/or with mono-resistance to isoniazid: A systematic review
and meta-analysis. PLoS Med. 2009;6: e1000150.
7. Latent Tuberculosis Infection: Updated and
Consolidated Guidelines for Programmatic Management. World Health
Organization; 2018.
8. Padmapriyadarsini C, Das M, Nagaraja SB, et al. Is
chemoprophylaxis for child contacts of drug-resistant TB patients
beneficial? A systematic review. Tuberc Res Treat. 2018: 3905890.
|
|
|
 |
|

