|
|
|
Indian Pediatr 2020;57: 1119-1123 |
 |
Effect of Umbilical Cord Milking vs
Delayed Cord Clamping on Venous Hematocrit at 48 Hours in
Late Preterm and Term Neonates: A Randomized Controlled Trial
|
|
Mukul Kumar Mangla, Anu Thukral, M Jeeva Sankar, Ramesh
Agarwal, Ashok K Deorari and VK Paul
From Division of Neonatology, Department of Pediatrics,
All India Institute of Medical Sciences, New Delhi, India.
Correspondence to: Dr Anu Thukral, Assistant Professor,
Department of Pediatrics, Newborn Health and Knowledge
Centre,
First Floor, New Private Ward, AIIMS, New Delhi 110 029,
India.
Email:
[email protected]
Received: November 03, 2019;
Initial review: January 20, 2020;
Accepted: September 08, 2020.
Trial Registration: CTRI/2016/11/007470
Published online: September 16, 2020;
PII: S097475591600248
|
|
Objective: To compare the effect of intact umbilical cord
milking (MUC) and delayed cord clamping (DCC) on venous
hematocrit at 48 (±6) hours in late preterm and term neonates
(350/7- 426/7 wk).
Study Design:
Randomized trial.
Setting and participants:
All late preterm and term neonates (350/7 - 426/7 wk) neonates
born in the labor room and maternity operation theatre of
tertiary care unit were included.
Intervention: We
randomly allocated enrolled neonates to MUC group (cord milked
four times towards the baby while being attached to the
placenta; n=72) or DCC group (cord clamped after 60
seconds; n=72).
Outcome: Primary
outcome was venous hematocrit at 48 (±6) hours of life.
Additional outcomes were venous hematocrit at 48 (±6) hours in
newborns delivered through lower segment caesarean section
(LSCS), incidence of polycythemia requiring partial exchange
transfusion, incidence of hyperbilirubinemia requiring
phototherapy, and venous hematocrit and serum ferritin levels at
6 (±1) weeks of age.
Results: The mean
(SD) hematocrit at 48 (±6) hours in the MUC group was higher
than in DCC group [57.7 (4.3) vs. 55.9 (4.4); P=0.002].
Venous hematocrit at 6 (±1) weeks was higher in MUC than in DCC
group [mean (SD), 37.7 (4.3) vs. 36 (3.4); mean
difference 1.75 (95% CI 0.53 to 2.9); P=0.005]. Other
parameters were similar in the two groups.
Conclusion: MUC leads
to a higher venous hematocrit at 48 (±6) hours in late preterm
and term neonates when compared with DCC.
Keywords: Anemia, Infant,
Placental redistribution, Transfusion.
|
P
lacental
transfusion provides sufficient iron reserves for the first
3 to 6 months of life; thus, preventing or delaying the
development of iron deficiency until the use of
iron-fortified foods is implemented [4]. Delayed cord
clamping (defined variably as clamping till cessation of
pulsations or up to 60-180 seconds) leads to improvement in
levels of hemo-globin and hematocrit at two months of age
[5]. However, universal application is limited in particular
due to obstetrician concerns for the risk of hypothermia,
and delay in initiation of resuscitation, when indicated
[6,7].
Umbilical cord milking, on the other
hand, involves milking the entire contents of the umbilical
cord towards the baby. Cut umbilical cord milking (umbilical
cord detached from placenta) limits the refilling of cord
from placenta, so less blood is likely to be transfused when
compared to intact umbilical cord milking (pushing the blood
toward the infant at least four times before clamping the
umbilical cord) [8-12].
Till date, two studies [13,14] have
evaluated the effect of delayed cord clamping and umbilical
cord milking in term neonates. In both studies, cut
umbilical cord milking was performed. Hence, we planned the
present study to evaluate the effect of intact umbilical
cord milking on venous hematocrit at 48 hours of age in late
preterm and term neonates when compared with delayed cord
clamping.
METHODS
This open labelled randomized trial was
conducted in the department of obstetrics and gynecology,
All India Institute of Medical Sciences, New Delhi from May
to September, 2016. All late preterm and term neonates (35 0/7
- 426/7 week)
were included in the study. Neonates with fetal hydrops,
major congenital malformation, Rh isoimmunization (Rh
positive neonate born to Rh negative mother with indirect
Coombs test (ICT) positive) [15], newborns born through
meconium stained liquor who were non-vigorous at birth
(defined by poor/no respiratory efforts, weak/no muscle tone
and heart rate less than 100 beats per minute, limp or
apneic or poor tone at birth) [16], forceps or vacuum
assisted delivery, and newborns born to HIV positive mother
(on enzyme-linked immunosorbent assay (ELISA) followed by
Western blot test for HIV) and maternal eclampsia (defined
as generalized seizure in pregnant females with
preeclampsia) [17] were excluded from the study. The study was
approved by the institutional ethics committee.
All eligible mothers admitted in the
labor room were screened for eligibility and enrolled, after
informed written consent. We allocated mothers using
computer generated random sequence to intact umbilical cord
milking group and delayed cord clamping group. Opaque
envelopes containing allocation group were serially
numbered, and sealed to conceal the identity. The sealed
envelope was opened by the nursing staff when the expectant
mother was wheeled inside the labor room. The intervention
written on slip was carried out by the obstetrics and
gynecology resident and pediatric resident team posted in
the labor room. Blinding of the clinicians was not possible
due to the obvious nature of intervention.
Intact umbilical cord milking (MUC) group:
The intact umbilical cord for its remaining accessible
length (nearly half of the total length) was milked four
times towards the baby by the residents on duty in the
obstetrics department, and then clamped. All the health
care providers (postgraduate residents of pediatrics and
obstetrics) were trained in a structured manner for intact
umbilical cord milking.
Delayed cord clamping (DCC) group:
Umbilical cord was clamped at least 60 seconds from the
time of delivery.
Time of all interventions was recorded by
a stopwatch and noted in the study form. In both the groups,
the baby was held at introitus after vaginal delivery, and
over mother’s thigh in caesarean delivery. After delivery,
the babies were kept with mothers unless they required
admission in the neonatal intensive care unit (NICU) for
standard indications. Gestational age was assigned based on
the last menstrual period. The appropriateness of
birthweight for gestational age was assigned by stan-dard
intrauterine growth chart [18]; weight less than 10th
centile and weight more than 90th centile being adjudged as
small for gestational age (SGA) and large for gestatio-nal
age (LGA), respectively [18]. Early breastfeeding was
encouraged in all babies as per standard guidelines. The
infants were evaluated at birth, and at the age of 24 hours
and then at 48 hours.
The primary outcome was venous hematocrit
evaluated at 48 (±6) hours. Additional outcomes were venous
hematocrit at 48 (±6) hours in newborns delivered by lower
segment caesarean section (LSCS), incidence of polycythemia
(defined as venous hematocrit greater than 65% at 48 (±6)
hours of life) [19] requiring partial exchange (PET),
incidence of hyperbilirubinemia requiring photo-therapy (as
per American Academy of Pediatrics (AAP) charts) [20],
venous hematocrit at 6 (±1) weeks, and levels of serum
ferritin at 6 (±1) weeks.
Venous sample was collected in micro-
capillaries for measurement of hematocrit, and an additional
1 mL sample was separately collected for serum ferritin
levels. Micro-capillaries were micro-centrifuged at a speed
of 9000 rpm for 5 minutes and analyzed with card reader for
hematocrit measurement. Serum bilirubin was assessed in
babies with clinical icterus by spectrophotometer (Apel BR
5100, APEL). Calibration of the spectrophotometer was done
at defined intervals, as recommended by the manufacturer and
phototherapy was instituted, if required. Serum ferritin
levels were evaluated with ELISA orgentech kit (analytical
sensitivity, 5 ng/mL; range of evaluated concentrations
5-1000 ng/mL).
Attendants were counseled by the
principal investi-gator to follow up at 6 (±1) weeks, which
coincided with their immunization visit. Hematocrit
evaluation and serum ferritin levels were evaluated at this
time point. On follow up, parents were asked about any
intercurrent illnesses since birth, and type and mode of
feeding (top fed, exclusively breastfed or predominantly
breastfed.
Venous hematocrit at 48 (±6) hours in a
previous study was 50% [10]. Anticipating that intact
umbilical cord milking will lead to at least a 5% absolute
increase in the hematocrit and assuming a standard deviation
(SD) of 7 in each group with power of 90% and alpha of 0.05,
we needed to enroll at least 42 neonates in each arm.
Considering an attrition rate of 40% on follow up, total
sample size was increased to 72 in each group.
Statistical analyses:
Statistical analyses were performed with Stata 11 (Stata
Corp LP). Baseline categorical variables were compared using
Chi-square or Fisher exact test, as appropriate, and whereas
continuous variables were compared using Student t-test. A
P-value of less than 0.05 was considered as significant. The
analysis was by the intention to treat.
RESULTS
A total of 375 babies were delivered
during the enrolment period, of which 144 babies fulfilled
the inclusion criteria and were enrolled (Fig. 1).
Baseline characteristics including maternal pregnancy
induced hypertension, gestational diabetes, gestational age
and birthweight were comparable between the two groups (Table
I). 72 neonates were enrolled to DCC and 72 neonates to
MUC group. Out of the 144 neonates, 118 (82%) completed the
trial at 6 (±1) weeks. There were no adverse events in
either group during the study period.
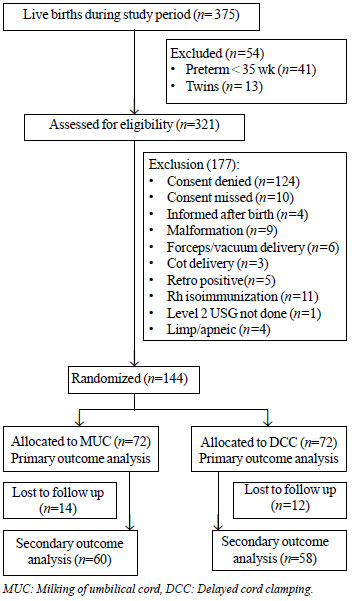 |
|
Fig. 1
Study flow chart.
|
Table I Baseline Maternal and Neonatal Characteristics of the Two Groups
|
Umbilical cord
|
Delayed cord
|
|
|
milking group |
clamping group |
|
(n=72) |
(n=72) |
|
Maternal characteristics |
|
|
|
Booked pregnancy |
72 (100) |
71 (99) |
|
Maternal age (y)* |
29.1 (4.2) |
28.3 (3.3) |
|
Lower segment caesarean section
|
31 (43) |
36 (50) |
|
Hemoglobin (g/dL)* |
11.7 (1.2) |
11.4 (1.1) |
|
Chronic hypertension |
7 (9.7) |
4 (5.5) |
|
Pregnancy induced hypertension |
3 (4.1) |
3 (4.1) |
|
Meconium stained liquor |
2 (2.7) |
6 (8.3) |
|
Intra uterine growth retardation |
1 (1.4) |
3 (4.1) |
|
Gestational diabetes mellitus |
13 (18.1) |
11 (15.2) |
|
Neonatal characteristics |
|
|
|
Gestation age (wk)* |
37.9 (1.0) |
37.8 (1.6) |
|
Male sex |
36 (50) |
36 (50) |
|
Small for date
|
1 (1.4) |
0 |
|
Large for date
|
10 (13.9) |
9 (12.5) |
|
Weight (g)* |
3038 (436) |
2909 (435) |
|
Use of any respiratory support |
3 (4.1) |
3 (4.1) |
|
Admission in NICU |
1 (1.4) |
2 (2.8) |
|
Time since cord clamp (s)*# |
12.9 (0.8) |
60 (0) |
|
Data depicted as n (%) or *mean (SD); All
P<0.05 except #P<0.01. |
The mean (SD) hematocrit at 48 (±6) hours
in the MUC group [57.7 (4.3)] was significantly higher than
the DCC group [55.9 (4.4)] [mean difference (MD)
1.7 (95% CI 0.21 to 3.1); P=0.002] (Table
II). Venous hematocrit in newborn delivered by caesarean
section at 48 (±6) hours was similar in the two groups.
Incidence of polycythemia was also similar in the two
groups. One neonate in each group required phototherapy.
Mean (SD) Venous hematocrit at 6 (±1) weeks was higher in
MUC than in DCC group [MD (95% CI) 1.75 (0.53 to 2.9); P=
0.005] (Table II). The levels of serum
ferritin were similar in the two groups (Table II
and Fig. 2).
Table II Primary and Secondary Outcome Variables in Late Preterm and Term Neonates in the Study
|
Parameter |
MUC group
|
DCC group |
Mean
|
|
(n=72) |
(n=72) |
difference |
|
|
|
(95% CI) |
|
Hematocrit at 48 (±6) h |
57.7
|
55.9
|
1.68
|
|
|
(4.3) |
(4.4) |
(0.21, 3.1) |
|
Secondary outcomes |
(n = 58) |
(n = 60) |
|
|
Hematocrit# |
37.7
|
36 9
|
1.7
|
|
(3.3) |
(3.4) |
(0.53, 2.9) |
|
Serum ferritin (ng/mL)# |
363.1 |
295.8 |
67.2
|
|
|
|
(-24.0, 158.5) |
|
Hyperbilirubinemia*
|
1 (1.4) |
1 (1.4) |
- |
|
Polycythemia^ |
0 |
2 (2.8) |
- |
|
Hematocrit at 48 (±6) h‡
|
57.4
|
56.4
|
1.02
|
|
|
(4.4) |
(4.8) |
(-1.2, 3.2) |
|
*Requiring phototherapy; #at 6 (±1) wk;
^requiring partial exchange transfusion; ‡Newborns
delivered by lower segment caesarean section,
n=31 in UCC and 36 in DCC group. |
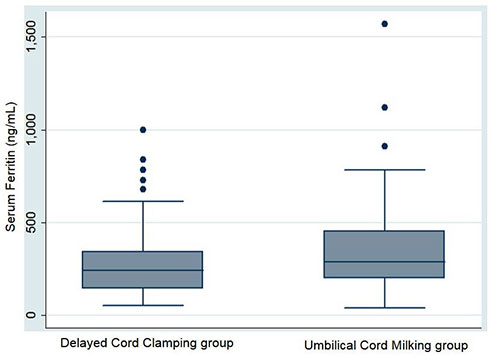 |
|
Fig. 2 Box-and-whisker
plot for serum ferritin at 6 (±1) weeks in neonates
in the delayed cord clamping and umbilical cords
milking groups.
|
DISCUSSION
This randomized trial compared intact
umbilical cord milking (MUC) with delayed cord clamping
(DCC) on venous hematocrit at 48 (±6) hours of life in late
preterm and term neonates. The hematocrit at 48 (±6) hours
and at 6 (±1) week was higher in the intact MUC group.
However, it was similar in the two groups in infants
delivered by LSCS. Other parameters including incidence of
polycythemia, incidence of hyperbiliru-binemia requiring
phototherapy and ferritin were similar in the two groups.
There are very few studies in late
preterm and term infants comparing MUC with DCC. Jaiswal,
et al. [13] evaluated the effect of MUC and DCC on
hematological parameters (serum ferritin and hemoglobin) at
6 (±1) weeks of life in term neonates. The packed cell
volume (PCV) at 48 (±6) hours and hemoglobin level at 6 (±1)
weeks postnatal age was similar in the two groups in
contrast to the results of the present study. Studies in
preterm infants comparing DCC suggest mixed results
[8,9,11,12]. The
cord vein contains nearly 20 mL of placental blood and
one-time umbilical cord milking (of cut segment of about 30
cm) can transfer nearly 18 mL/kg of blood to the newborn
[9,21,22]. The newborn is likely to get more blood if the
cord segment is intact, since this allows subsequent
refilling of cord from placenta explaining the higher
hematocrit at 48 (±6) hours and higher hemoglobin at 6 (±1)
weeks seen in the present study.
We observed no difference in the
hematocrit in MUC and DCC group in neonates delivered by
LSCS. A recent study by Katheria, et al. [11] in
preterm neonates delivered by cesarean delivery suggested a
higher hemoglobin (within the first 24 hours) in MUC group.
Infants delivered by cesarean section have a lower
circulating red cell volume due to the anesthetic and
surgical interventions which interfere with active uterine
contraction, thus leading to more blood volume remaining in
placenta and hence a lower hematocrit
[22]. However, we did not evaluate the
hematocrit at birth or within 24 hours.
We did not observe any difference in the
incidence of hyperbilirubinemia requiring phototherapy or
the incidence of polycythemia at 48 (±6) or any difference
in serum ferritin at 6 (±1) weeks. These findings have been
previously reported [8,9,12,16].
Our study is the first study in late
preterm and term neonates where intact umbilical cord
milking (milking done with umbilical cord attached to
placenta) was compared to delayed cord clamping for
evaluation of hematological parameters. This trial ensured
appropriate allocation concealment. The outcome assessors
and laboratory team were blinded to the intervention arm. We
had a follow up rate of 82%. Our study had some limitations
too. A longer follow-up till at least 6 to 12 months is
desirable to establish whether the initial advantage in
hematocrit also translates into gains in infancy and early
childhood, which we did not plan.
Umbilical cord milking leads to higher
venous hematocrit at 48 (±6) hours when compared with
delayed cord clamping in late preterm and term neonates,
however long-term effects of milking need to be further
evaluated.
Ethics clearance: Institute Ethics
Committee, AIIMS; No. IECPG/197/24.02.2016, RT-10, dated
March 30, 2016.
Contributors: MKM: protocol
development, study implementation, data management and
writing the manuscript; AT, MJS: development of the protocol
and supervised implementation of the study and contributed
to writing of the manuscript and did data analysis; VKP,
AKD, RA: protocol development, and provided critical inputs
in manuscript writing. All authors approved the final
version of manuscript, and are accountable for all aspects
related to the study.
Funding: None; Competing
interests: None stated.
|
WHAT IS ALREADY KNOWN?
•
Delayed cord
clamping leads to improvement in levels of
hemoglobin and hematocrit at two months of age.
WHAT THIS STUDY ADDS?
•
Umbilical cord milking leads to higher venous
hematocrit at 48 (±6) hours when compared with
delayed cord clamping in late preterm and term
neonates
•
Intact cord milking does not result in neonatal
hyperbilirubinemia or symptomatic polycythemia as
compared to delayed cord clamping.
|
REFERENCES
1. Lozoff B. Iron and learning potential
in childhood. Bull N Y Acad Med. 1989;65:1050-66.
2. Stevens GA, Finucane MM, De-Regil LM,
et al. Global, regional and national trends in
hemoglobin concentration and pre-valence of total and severe
anemia in children and pregnant and non-pregnant women for
1995-2011: A systemic analysis of population representative
data. Lancet Glob Health. 2013;1:e16-e25.
3. International Institute for Population
Sciences (IIPS) and Macro International. 2007. National
Family Health Survey (NFHS-3), 2005–06: India: Volume I.
IIPS. Available from: https://dhsprogram.com/pubs/pdf/FRIND3/FRIND3-Vol1AndVol2.pdf.
Accessed Jan 10, 2018.
4. WHO. Guideline: Delayed Umbilical Cord
Clamping for Improved Maternal and Infant Health and
Nutrition Outcomes. World Health Organization; 2014.
Available from: https:
www.who.int/nutrition/publications/guide lines/cord_clamping/eng.pdf.
Accessed January 10th 2018.
5. Beyond survival: integrated delivery
care practices for long-term maternal and infant nutrition,
health and development. II ed. PAHO, 2013. Available from:
https://www.who.int/nutrition/publications/infantfeeding/Beyond
Survival2ndeditionen.pdf?ua=1. Accessed Jan 20, 2018.
6. Jelin AC, Kuppermann M, Erickson K,
et al. Obstetricians’ attitudes and beliefs regarding
umbilical cord clamping. J Matern Fetal Neonatal Med.
2014;27:1457-61.
7. Boere I, Smit M, Roest AA, et al.
Current practice of cord clamping in the Netherlands: a
questionnaire study. Neonatology. 2015; 107:50-5.
8. Hosono S, Mugishima H, Fujita, et
al. Umbilical cord milking reduces the need for red cell
transfusions and improves neonatal adaptation in infants
born at less than 29 weeks’ gestation: A randomized
controlled trial. Arch Dis Child Fetal Neonatal Ed.
2008;93:F14-9.
9. Rabe H, Jewison A, Alvarez RF, et
al. Milking compared with delayed cord clamping to
increase placental transfusion in preterm neonates: A
randomized controlled trial. Obstet Gynecol.
2011;117:205-11.
10. Owen DA, Mercer JS, Oh W. Umbilical
cord milking in term infants delivered by caesarean section:
A randomized controlled trial. J Perinatol. 2012;32:580-84.
11. Katheria AC, Truong G, Cousins L,
Oshiro B, Finer NN. Umbilical cord milking versus delayed
cord clamping in preterm infants. Pediatrics.
2015;136:61-69.
12. Shirk S, Manolis S, Lambers D, Smith
K. Delayed clamping vs. milking of umbilical cord in
preterm infants: A randomized control trial. Am J Obstet
Gynecol, 2019; 220:e1-8.
13. Jaiswal P, A Upadhyay, Gothwal S,
et al. Comparison of two types of intervention to
enhance placental redistribution in term infants:
Rando-mized control trial. Eur J Pediatr. 2015;17:1159-67.
14. Yadav AK, Upadhyay A, Gothwal S,
Dubey K, Mandal U, Yadav C. Comparison of three types of
intervention to enhance placental redistribution in term
newborns: Randomized control trial. J Perinatol.
2015;35:720-24.
15. Moise KJ. Management of rhesus
isoimmunization in pregnancy. Obstet Gynecol: 2008;
112:164-76.
16. Weiner GM, Zaichkin J. Textbook of
neonatal resusci-tation.7th edition: American Academy of
Pediatrics; 2016.p.12.
17. Fauvel JP. Hypertension during
pregnancy: Epidemiology, definition. Presse Med.
2016;45:618-21.
18. Singhal PK, Paul VK, Deorari AK,
Singh M, Sunderam KR. Changing trends in intrauterine growth
curves. Indian Pediatr. 1991;28:281-83.
19. Ramamurthy RS, Brans WY. Neonatal
polycythemia. Criteria for diagnosis and treatment.
Pediatrics. 1980;97:118-20.
20. American Academy of Pediatrics
Subcommittee on Hyperbilirubinemia: Management of
Hyperbilirubinemia in the Newborn Infant 35 or More Weeks of
Gestation. Pediatrics. 2004;114:297-316.
21. Blood. In: Haneef SM, Maqbool
S, Arif MA, eds. Text book of Paediatrics.
International Book Bank; 2004.p.545.
22. Aladangady N, McHugh S, Aitchison TC, Wardrop CA,
Holland BM. Infant’s blood volume in a controlled trial of
placental transfusion at preterm delivery. Pediatrics.
2006;117:93-8.
|
|
|
 |
|

