|
|
|
Indian Pediatr 2019;56: 1020-1024 |
 |
Seasonal Variation in Serum 25-hydroxy
Vitamin D and its Association with Clinical Morbidity in Healthy
Infants from Northern India
|
|
Ranadeep Ray 1,
Aashima Dabas1,
Dheeraj Shah1,
Rajeev Kumar Malhotra2,
SV Madhu3 and
Piyush Gupta1
From Departments of 1Pediatrics,
2Biostatistics and Medical Informatics, and 3Endocrinology,
University College of Medical Sciences and Guru Teg Bahadur Hospital,
Dilshad Garden, Delhi, India.
Correspondence to: Dr Piyush Gupta, Professor,
Department of Pediatrics, UCMS and GTB Hospital, Dilshad Garden, Delhi
110 095, India. Email:
[email protected]
Received: January 14, 2019;
Initial review: February 19, 2019;
Accepted: October 04, 2019.
|
|
Objective: To evaluate the
seasonal change in serum 25-hydroxyvitamin D (25-OHD) level in healthy
infants and to relate it to common childhood morbidities. Methods:
72 healthy breastfed infants residing in Delhi were enrolled at the
end of summer and followed till the end of winter [mean (SD) duration
200 (10) d]. Serum 25-OHD was estimated at baseline and follow-up.
Infants were monitored for common childhood diseases. Results:
Mean (SD) serum 25-OHD level was lower at the end of winter (20.7 (8.02)
ng/mL) than summer (22.9 (8.70) ng/mL) [mean difference (95% CI) –2.14
ng/mL (–3.36, –1.06), P<0.001). The seasonal distribution of
children according to vitamin D status in summer and winter - Deficient
(15.3%, 12.5%), Insufficient (19.4%, 30.6%) and Sufficient
(65.3%, 56.9%), respectively was comparable P=0.17). The
morbidity profile remained unaffected by change in vitamin D status from
summer to winter. Conclusions: Seasonal changes in vitamin D
levels do not have significant clinical effect or effect on overall
vitamin D status in apparently healthy infants from North India. This
may have implications for results of population surveys for vitamin D
status, irrespective of the season when they are conducted.
Keywords: Extra-skeletal, Hypovitaminosis D,
Outcome, Risk, Summer, Winter.
|
|
S
unlight is the main source of endogenous
synthesis of vitamin D in the human body. Vitamin D deficiency remains a
widely prevalent disorder in Northern India, a region which lies in the
temperate belt of the earth and receives adequate sunlight [1]. Vitamin
D levels are reported to be lower during winters [2], postulated due to
increasing obliquity (Zenith angle) of sun rays reaching the earth’s
surface [3]. Seasonal variation may have important implications on
prevalence of vitamin D deficiency and cutoffs used to define vitamin D
deficiency across different latitudes [4]. People living at northern
latitudes have lower vitamin D levels during winters with minimal
sunlight exposure, thereby necessitating vitamin D fortification or
supplementation during winters [5].
There has been renewed interest in the effect of
environment on human health. Global warming is causing climatic changes
around the world such that summers have become harsher (leading to less
outdoor activity), and winters have become pleasant (allowing more time
outdoors) thus affecting duration of exposure to sunlight in each of the
seasons [6]. We hypothesized that the seasonal variation in vitamin D
status may have declined to an insignificant level, especially in
regions with abundant sunshine. The study was conducted to measure the
seasonal variation in serum 25-OHD levels in a sample of infants
attending immunization clinics of a tertiary-care hospital in Delhi. The
secondary objective was to determine whether seasonal change in vitamin
D nutriture has any implication for common childhood morbidities.
Methods
This longitudinal observational study was conducted
in the Departments of Pediatrics and Endocrinology at a tertiary-level
hospital in Delhi (28.7 oN,
77.1oE) from May 2016 till
April 2017. The study was approved by the Institutional Ethics
Committee. Written informed consent was obtained from the parents for
participation.
Apparently healthy infants aged 9-10 months were
enrolled at the end of peak summer season in Delhi (first two weeks of
July) during their measles immunization visit. Infants were considered
eligible if they had a birthweight more than 2.5 kg and were born at
term gestation, were predominantly breastfed till 6 months of age, and
whose families resided within 5 km of the hospital. Any infant with
congenital malformations, history of seizures, clinical evidence of
rickets, chronic systemic disorder, past hospitalization, or with
history of receiving calcium or vitamin D supplements or mega dose
(>60000 IU) in last six months was excluded. Infants receiving vitamin
D-fortified milk or artificial formula were also excluded.
The relevant maternal details for any antenatal
complication and intake of calcium or vitamin D supplements during
antenatal period and lactation were recorded at the time of enrolment.
The birth details and feeding history were also recorded. Family history
of any skeletal disease was noted. Weight, length and head circumference
of the infant were measured at baseline and interpreted as per WHO
Growth Standards [7].
Baseline venous blood sample (2 mL) was obtained at
enrolment; serum was separated by centrifugation and stored at -20°C.
The infants were followed up monthly either during outpatient visits or
telephonically for next 7 months till completion of winter season (first
two weeks of February). During follow up period, any episodes of febrile
illness, acute respiratory infection, diarrhea, seizure, or meningitis
were recorded. Children were examined for any signs of rickets at
baseline and during follow-up based on clinical examination and
radiological findings on wrist X-ray. Morbidities were defined as
follows: Febrile illness: Axillary temperature
£38°C for >3 days
[8]; Diarrhea: Passage of 3 or more loose or liquid stools per
day (or more frequent passage than is normal for the individual) [9];
Acute respiratory infection: Fever with cough, with or without fast
breathing [10]; Meningitis: Acute onset of fever (usually >38.0°C
axillary), headache, and one of the following signs: neck stiffness,
altered consciousness or other meningeal signs [11].
A second blood sample was collected at the end of 7
months which coincided with the end of winter season and
Measles-Mumps-Rubella (MMR) vaccination between 15-18 months of age.
Paired serum samples were analyzed for 25-OHD, calcium, phosphorus and
alkaline phosphatase. Serum 25-OHD was estimated by Radio immunoassay
technique (RIA) with kits manufactured by Beckman Coulter, USA (total
imprecision ³10.0%
CV at > 15.0 ng/mL, and total standard deviation (SD)
³1.5 ng/mL at
³15.0 ng/mL). Vitamin
D status for serum 25-OHD values was interpreted as per the following
cut-offs: sufficient >20 ng/mL, insufficient 12-20 ng/mL, and deficient
<12 ng/mL [12]. The vitamin D status based on absolute serum 25-OHD
values was assessed separately during summer and winter for each infant.
Assuming a 25% drop in serum vitamin D levels during
winter from summer levels as clinically significant and mean serum
25-OHD level in Indian infant during summer as 16.96 ng/mL [13] with
power of study as 80% and one-tailed
a error 0.05, the
sample size was calculated as 67 (SD of change is 14 ng/mL). Assuming an
attrition rate of 10%, total sample required was 75.
Statistical analyses: Seasonal changes in
laboratory parameters were compared with paired t-test. McNemar-Bowker
test was applied to compare the vitamin D status between summer and
winter. The mean seasonal change in serum 25-OHD between vitamin D
deficient, insufficient, and sufficient children was analyzed by Kruskal-Wallis
test (for overall difference) followed by pair-wise comparison by
Mann-Whitney test for differences between any two categories (with
Bonferroni correction); since the change in serum 25-OHD was not
normally distributed. For the secondary outcome variable, participants
were stratified in three groups according to change in vitamin D status
during winter from summer (improved, no change, and reduced). Kruskal-Wallis
test was applied to compare the distribution of morbidity episodes among
these three groups. P value less than 0.05 was considered as
significant.
Results
A total of 98 infants were approached for enrollment,
out of which 75 infants (47 boys, mean age 9.3 months) were enrolled.
Mean (SD) birthweight was 2.9 (0.25) kg and weight-for-age SDS was 0.59
(0.63). Mothers of 55 (73.3%) infants took antenatal calcium
supplementation (500 mg tablet single daily dose; mean (SD) duration of
46.9 (26.3) day). All infants received home based complementary feeds
without vitamin D fortification after six months of age. Of these, 72
were available at the end of follow up [mean (SD) duration, 200 (10) d].
History of sun exposure was present in 73 children and two children had
no history of regular sun exposure. The duration of sun exposure ranged
from 30 min to 3.5 hours per week without any significant seasonal
variation (1.52 hr in winters and 1.5 hr in summer). Only 17 (22.7%)
families had history of practices promoting sun exposure. Mean
(SD) serum 25-OHD levels in summer and winter were 22.9 (8.70) and 20.7
(8.02) ng/mL, respectively [mean difference –2.14 (range -11 to +8 ng/mL)
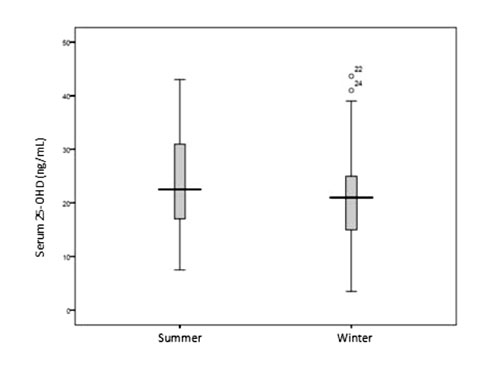
(95% CI: –3.36, –1.06); P<0.001] (Fig. I). The
average drop was 9.34% in winter. Despite this, the distribution of
children according to vitamin D status categories (deficient,
insufficient, and sufficient) was comparable between summer and winter (P=0.168)
(Table I). The mean (SD) change in serum 25-OHD (from
summer to winter) among vitamin D deficient, insufficient, and
sufficient children was -0.25 (3.69), -0.08 (4.46) and 3.36 (4.6) ng/mL,
respectively (P=0.005); among groups, difference was significant
between deficient and sufficient children (P=0.03). There was no
significant difference in sun exposure behavior between three groups.
 |
|
Fig. 1 Box-Whisker plot of serum
25-OHD (ng/mL) during summer and winter season.
|
TABLE I Seasonal Variation in Vitamin D Status of Infants
|
Vitamin D status |
Winter |
|
|
Deficient |
Insufficient
|
Sufficient |
Total |
|
Summer |
Deficient
|
8 |
3 |
0 |
11 (15.3%) |
|
Insufficient |
1 |
9 |
4 |
14 (19.4%) |
|
Sufficient |
0 |
10 |
37 |
47 (65.3%) |
|
Total
|
9
|
22 |
41 |
72 |
|
|
(12.5%) |
(30.6%) |
(56.9%) |
|
|
Vitamin D status: deficient (<12ng/mL), insufficient (12-20
ng/mL),
|
Mean (SD) serum calcium level was comparable between
summer and winter (9.2 (1.41) and 8.9 (1.51) mg/dL, respectively P=0.11).
Similarly, mean (SD) serum alkaline phosphatase levels in summer and
winter were comparable (291.6 (132.8) and 297.4 (145.7) IU,
respectively; P=0.57). Mean (SD) serum phosphorus level in summer
was significantly lower than winter (4.1 (1.21) and 4.7 (1.46) mg/dL,
respectively; P=0.009).
During follow-up, 69/72 (96%) children had at least
one episode of illness and 23 children (31.9%) were hospitalized. The
morbidities included: febrile illness (57, 79.2%), acute respiratory
infection (49, 68.1%), diarrhea (31, 43.1%), seizures (9,12.5%) and
meningitis (2, 2.7%). Additional 10 children became vitamin D
insufficient in winter from summer (Table I). Total 18
children changed their vitamin D status categories; 7 improved while 11
shifted to a lower category. There was no significant difference in
various morbidities and total morbidities median count among three
groups according to change in vitamin D status category (improved, no
change, and reduced) between summer and winter (Table II).
Serum 25-OHD across either did not have any influence on incidence of
morbidity (data not shown). Four children developed rickets and one
developed hypocalcemic seizures during follow-up (mean serum 25-OHD
15.5, 18.0, 20.2 and 12 ng/mL, and 10.8 ng/mL, respectively).
TABLE II Association of Seasonal Change in Vitamin D Status with Common Morbidities
|
Morbidity |
Change in vitamin D status from summer to winterP value |
|
|
Improved (n=7) |
No change (n=54) |
Reduced (n=11) |
|
|
Febrile illness |
1.0 (1.0-1.0) |
1.0 (1.0-2.0) |
1.0 (1.0-1.0) |
0.332 |
|
Acute respiratory infection |
1.0 (0.0-1.0) |
1.0 (0.0-1.25) |
1.0 (0.0-1.0) |
0.942 |
|
Diarrhea |
0.0 (0.0-1.0) |
0.0 (0.0-1.0) |
0.0 (0.0-1.0) |
0.246 |
|
Total illnesses |
1.0 (1.0-2.0) |
2.0 (1.0-3.0) |
2.0 (1.0-3.0) |
0.5 |
|
Values represent median number (Inter-quartile range) of
episodes of morbidity. |
Discussion
We documented lower mean serum 25-OHD levels in
winter than summer in healthy infants from northern India. However, this
was not associated with any significant change in the overall vitamin D
nutritional status of the population, especially among those with
borderline or low serum 25-OHD levels. Further, there was no significant
association between seasonal change in vitamin D levels with the overall
occurrence of extraskeletal childhood morbidities
Higher vitamin D levels during summer have been
reported among children and adults in temperate countries; the
difference ranging from 5.4 to 15 ng/mL [2,14]. We, in an earlier study,
documented a significant correlation of serum vitamin D levels with
sunlight exposure in infants [15]. To the contrary, Jain, et al
[6] did not find significant seasonal difference in infants’ serum
vitamin D levels between summer and winter. Observations of
insignificant seasonal difference or higher winter serum 25-OHD values
in young children have been similarly reported by other authors [16]. A
longer sun exposure of children during winter than summer due to harsher
summer temperatures was postulated to increase winter vitamin D levels
[6]. The winter serum 25-OHD levels were only marginally lower than
summer levels in the present study. It is possible that harsher summers
in Delhi (maximum temperature 46 oC)
resulted in avoidance of sunlight exposure by the participants leading
to only a small difference in vitamin D levels between summer and winter
despite Delhi lying in a temperate zone. This may also support the
higher summer vitamin D seen in temperate countries which receive
minimal sunlight during winters [2,14]. It was interesting to note that
vitamin D deficient children showed little seasonal variation as
compared to those with vitamin D sufficiency. It is probable that low
serum 25-OHD levels in the former group did not reach statistical
significance and that factors other than sunlight exposure maybe more
important in maintaining vitamin D status, following a compromise in the
body vitamin D status. It is possible that compensatory mechanisms such
as increased gastrointestinal absorption of vitamin D are more active in
vitamin-D deficient children, which prevent further reduction in vitamin
D status of the body following a reduction in sunlight exposure that
might have happened in winter.
We did not observe any association between seasonal
variation in vitamin D nutriture and common extra-skeletal childhood
morbidities, like earlier reports [17,18]. Observational studies have
reported an inverse association between serum vitamin D levels and risk
of childhood infections [19,20]. However, supplementation of vitamin D
did not significantly improve disease outcomes in clinical trials [21].
Our study had some limitations like lack of an
objective record of sun-exposure, lack of availability of maternal
vitamin D levels, and non-availability of spectroscopy for vitamin D
estimation. The sample size for subgroup analysis, for childhood
morbidities based on vitamin D levels was also small.
To conclude, the present study reported a slightly
lower winter level of serum vitamin D among Indian infants than in
summer. The change was not associated with common extraskeletal
morbidities, thereby lacking clinico-epidemiological relevance. These
results have important implications when considering season as a
confounding factor in population surveys of vitamin D status. As the
vitamin D status categories do not change much during summer and winter,
the surveys to estimate prevalence of vitamin D deficiency should remain
valid, irrespective of season in which they are conducted. On the other
hand, seasonality may be of importance in children having normal vitamin
D status, and studies to estimate normal levels of vitamin D among
healthy children should consider the season of data collection.
Contributors: PG,DS.AD,RD,DS,RKM, study
was conceived; SVM: contributed to the study design and writing the
proposal for research; RD: data collection was handled; AD, DS, SVM and
PG. SVM: data collection was handled, supervised and also supervised the
laboratory work" up of vitamin D status; RKM,PG: statistical analysis was
carried; AD,RD,PG: literature search was conducted; RD,AD: initial draft
of the manuscript was written; PG,DS,SVM,RKM: initial draft of the
manuscript was written which was edited and refined by provided critical
inputs to the draft manuscript. The manuscript was seen and approved by
all authors.
Funding: None; Competing interest: None
stated.
|
What This Study Adds
• There is a small seasonal variation in
serum 25 OHD in Northern India which neither affects the overall
vitamin D status of the infants nor relates to common childhood
illnesses.
|
References
1. Holick MF. Environmental factors that influence
the cutaneous production of vitamin D. Am J Clin Nutr. 1995;61:638S-45S.
2. Rajakumar K, Hollick MF, Jeong K, Mooreet CJ.
Impact of season and diet on vitamin D status of African, American and
Caucasian children. Clin Pediatr. 2011;50:492-502.
3. Wacker M, Holick MF. Sunlight and vitamin D: A
global perspective for health. Dermatoendocrinol. 2013;5:51-108.
4. Michaëlsson K, Wolk A, Byberg L, Mitchell A, Mallmin
H, Melhus H. The seasonal importance of serum 25-hydroxyvitamin D for
bone mineral density in older women. J Intern Med. 2017;281:167-78.
5. Huotari A, Herzig KH. Vitamin D and living in
northern latitudes–an endemic risk area for vitamin D deficiency. Int J
Circumpolar Health. 2008;67:164-78.
6. Jain V, Gupta N, Kalaivani M, Jain A, Sinha A,
Agarwal R. Vitamin D deficiency in healthy breastfed term infants at 3
months and their mothers in India: Seasonal variation and determinants.
Indian J Med Res. 2011;133:267-73.
7. WHO Multicentric Growth Reference Study Group. WHO
child growth standards based on length/ height, weight and age. Acta
Pediatr. 2006;Supp 450:76-85.
8. American College of Emergency Physicians. Clinical
Policy for Children Younger Than Three Years Presenting to the Emergency
Department with Fever: ACEP Guidelines. Ann Emerg Med. 2003;42:530-45.
9. World Health Organization. The Treatment of
Diarrhoea: A Manual for Physicians and Other Senior Health
Workers. Geneva: World Health Organization; 2005. Available at URL: http://www
.who.int/child_adolescent_ health/documents/9241593180/en/index.html.
Accessed March 14, 2016.
10. World Health Organization. Acute Respiratory
Infections in Children: Case Management in Small Hospitals in Developing
Countries, a Manual for Doctors and Other Senior Health Workers.
Available at URL: http://www.who.int/iris/handle/10665/61873.
Accessed March 14, 2016.
11. van de Beek, Cabellos C, Dzupova O, Esposito S,
Klein M, Kloek AT, et al. ESCMID guideline: Diagnosis and
management of acute bacterial meningitis. Clin Microbiol
Infect. 2016;22:S37-62.
12. Institute of Medicine (US) Committee to review
dietary reference intakes for vitamin D and calcium. In: Ross AC,
Taylor CL, Yaktine AL, Del Valle HB, editors. Dietary reference intakes
for calcium and vitamin D. Washington (DC): National Academies Press
(US); 2011.
13. Agarwal N, Faridi MMA, Aggarwal A, Singh O.
Vitamin D status of term exclusively breastfed infants and their mothers
from India. Acta Paediatr. 2010;99:1671-4.
14. Rockell JE, Green TJ, Murray C, Susan J. Season
and ethnicity are determinants of serum of serum 25(OH)D concentration
in New Zealand children aged 5-14 yr. J Nutr. 2005;135: 2602-8.
15. Meena P, Dabas A, Shah D, Malhotra RK, Madhu
SV, Gupta P. Sunlight exposure and vitamin D status in breastfed
infants. Indian Pediatr. 2017;54:105-11.
16. Tiwari L, Puliyel JM, Upadhyaya P, Taneja V,
Mittal R. Vitamin D levels in slum children in Delhi. Indian Pediatr.
2004~41:1076-7.
17. Sudfeld CR, Manji KP, Smith ER, Aboud S, Kisenge
R, Fawzi WW, et al. Vitamin D deficiency is not associated with
growth or the incidence of common morbidities among Tanzanian infants. J
Pediatr Gastroenterol Nutr. 2017;65:467-74.
18. Chowdhury R, Taneja S, Bhandari N, Sinha B, Upadhyay
RP, Bhan MK, et al. Vitamin-D deficiency predicts infections in
young north Indian children: A secondary data analysis. PLoS
One. 2017;12:e0170509.
19. Jarti T, Ruuskanen O, Mansbach JM, Vuorinen T,
Camargo CA. Low serum 25-hydroxyvitamin D levels are associated with
increased risk of viral co-infections in wheezing children. J Allergy
Clin Immunol. 2010;126:1074-6.
20. Asilioglu N, Cigdem H, Paksu MS. Serum vitamin
D status and outcome in critically ill children. Indian J Crit Care Med.
2017;21:660-4.
21. Gupta P, Dewan P, Shah D, Sharma N, Bedi N, Kaur
IR, et al. Vitamin D supplementation for treatment and prevention
of pneumonia in under-five children: A randomized double-blind placebo
controlled trial. Indian Pediatr. 2016;53:967-76.
|
|
|
 |
|

