|
|
|
Indian Pediatr 2019;56:1011-1016 |
 |
Propofol versus
Fentanyl for Sedation in Pediatric Bronchoscopy: A Randomized
Controlled Trial
|
|
PKG Gunathilaka, Kana Ram Jat, Jhuma Sankar, Rakesh Lodha and SK
Kabra
From Department of Pediatrics, All India Institute of Medical
Sciences, New Delhi, India.
Correspondence to: Prof SK Kabra, Department of Pediatrics, All India
Institute of Medical Sciences, New Delhi 110 029, India,
Email: [email protected]
Received: September 14, 2018;
Initial review: July 10, 2019;
Accepted: October 05, 2019.
|
Objectives: To compare propofol and fentanyl to
induce conscious sedation in children undergoing flexible bronchoscopy.
Study design: Randomized controlled trial.
Setting: Pediatric Pulmonology division at a
tertiary care center in Delhi, India.
Participants: Children aged 3-15 years who
underwent flexible bronchoscopy.
Intervention: Children received either
intravenous propofol 1 mg/kg administered as a slow bolus over 1 minute
followed by 2 mg/kg/hour infusion, or intravenous Fentanyl 2 µg/kg
administered as a slow bolus over one minute.
Outcomes: Primary outcome was time to achieve
conscious sedation (Ramsay score 3). Secondary outcomes were need for
adjuvant midazolam, physician satisfaction, level of cough, recovery
features, and side-effects in the groups.
Results: 53 children (propofol 27, fentanyl 26)
were enrolled in the study. The mean (SD) time taken to achieve Ramsay
score 03 was lower in propofol than fentanyl [15.7 (4.4) s vs 206
(55) s, P<0.001]. Propofol arm had significantly higher physician
satisfaction, less requirement of adjuvant midazolam, less coughing and
faster regain of full consciousness. There was no difference in drug
side-effects between the groups.
Conclusion: Propofol has a shorter sedation
induction time, less coughing during procedure, less recovery time, and
better physician satisfaction compared to fentanyl for flexible
bronchoscopy in children.
Keywords: Conscious sedation, Endoscopy,
Ramsay score, Visual analog scale.
Trial registration: CTRI/2016/09/007307
|
|
F
lexible video bronchoscopy with its ancillary
procedures (broncho-alveolar lavage, transbronchial biopsy, bronchial
washings, bronchial brushing and transbronchial needle aspiration) are
well established diagnostic techniques, while endoscopic bronchial
ultrasound and auto fluorescence bronchoscopy allow advanced evaluation
of mediastinal, endobronchial and parenchymal lesions [1]. General
anaesthesia was the preferred mode of anaesthesia for bronchoscopy in
pediatric practice; however, according to modern practice, conscious
sedation is the most routine anaesthetic measure utilized by pediatric
bronchoscopists [2]. It is safer and economical than deep sedation or
general anaesthesia [3].
Chloral hydrate, benzodiazepines such as midazolam
and opioids such as fentanyl are the most common sedative medications
used in pediatric procedure room [4]. In pediatric bronchoscopy,
fentanyl is utilized widely, alone or in combination with other
medications [5]. Propofol is being used increasingly in pediatric
bronchoscopy procedures in recent times [6,7]. In addition, procedural
sedation administration is done inside procedure room by physicians
instead of anaesthetist, in many centers [8].
Propofol has been used in combination with fentanyl
in pediatric bronchoscopy as a sedative strategy and it has been shown
to be better than volatile agents [9]. Propofol and fentanyl have been
used in isolation with good outcomes [5,7]. While multiple combinations
have been compared in different studies [6,9], propofol and fentanyl
have not been compared with each other. There is a need to establish a
safe and effective sedation regimen for paediatric bronchoscopy and
close a gap in the knowledge. Therefore, in this study, we compared the
time required to induce the level of conscious sedation to achieve
Ramsay score 3 [10] after administration of sedative medication (propofol
or fentanyl) in children undergoing fibreoptic bronchoscopy.
Methods
All children, 3 to 15 years of age, admitted to a
tertiary-care hospital in northern India for flexible bronchoscopy in
the division of Pediatric Pulmonology between 1 st
November, 2016 and 1st May,
2017 were screened for eligibility for the study. Children with any of
the following were excluded: previous untoward reaction for medications
used for sedation, children with history of lipid allergy,
cardiovascular instability (needing inotropic support), oxygen
dependency at the time of enrolment, oxygen saturation <90% at the time
of enrolment, encephalopathy or impaired consciousness, evidence of
acute or chronic liver disease, children who are already on any sedative
medication including antiepileptic drugs, or any intervention which
would interfere with outcome, and contraindications to use these
medications. Children were enrolled after written informed consent was
obtained from parents or legally authorized representative. The trial
was approved by the Institutional Ethics Committee.
As there is a lack of pediatric data to compare time
to achieve conscious sedation with propofol and fentanyl, we did an
interim analysis after 30 patients to calculate sample size. Mean time
to achieve Ramsay score 3 in fentanyl group was 194.8 (62.12) seconds.
We assumed that propofol would decrease this time by 25%. To detect this
difference with 95% confidence and 80% power, the calculated sample size
was 52 children (26 per group).
Children were randomized using computer-generated
block randomization with variable block sizes, performed by a person not
involved in the study. The respective randomization lists were kept in
sequentially numbered, sealed, opaque envelopes for allocation
concealment. All the envelopes were kept inside the bronchoscopy room in
a locker and envelopes were taken out according to the serial number and
were opened by bronchoscopy nurse-in-charge and the arm was documented
against the serial number in a separate paper. Selected intervention was
given by resident in-charge of the bronchoscopy room. Due to the
apparent difference in the colour of the medications in this study, the
investigator and residents were not blinded to the study arm. However,
the assignment was not disclosed to the patient or the bronchoscopist.
For children randomized to arm 1, intravenous
propofol 1 mg/kg (maximum of 50 mg) was administered as a slow bolus
over 1 minute followed by 2 mg/kg/hour infusion for maximum of 15
minutes or till end of the procedure, whichever occurred earlier. One
percent propofol (10 mg/mL) was used for slow bolus and propofol was
diluted with 5% dextrose to make dilution of 2 mg/mL for infusion. For
children randomized to arm 2, intravenous Fentanyl 2 µg/kg (maximum of
100 µg) was administered as a slow bolus over one minute. Fentanyl was
diluted with normal saline to make it 10 µg/mL.
The child’s oxygen saturation, pulse rate and
respiratory rate were documented and monitored during the procedure and
thereafter, by a designated health worker, till recovery from sedation.
Free flow oxygen at flow rate of 10 L/min was administered through a
tube (from the nostril other than the one used for inserting the
bronchoscope). Standard resuscitation facilities were available during
the procedure and till recovery from sedation. IV propofol or fentanyl
was administered according to the selected arm and a digital stop watch
was started at the end of administration of the respective bolus
medication. The stop watch reading was documented in seconds with the
achievement of spontaneous closure of eyes (Ramsay Score 3), by the
principal investigator [10].
If Ramsay score of 3 could not be achieved at end of
180 seconds of end of IV propofol/fentanyl bolus, a dose of IV midazolam
0.1 mg/kg (maximum dose of 5 mg) was administered and child observed for
1 minute; in case of failure, second dose (0.1 mg/kg) was administered
and child observed for another 1 minute. At the end of 5 minutes, if
sedation had been not achieved, it was considered as sedation failure.
In addition, midazolam was administered at a dose of 0.1 mg/kg (maximum
dose of 5 mg) bolus at a time up to maximum of two doses, for those who
had inadequate sedation to continue procedure irrespective of the arm.
Number of midazolam boluses was documented.
The video recording of bronchoscopy was started at
the beginning of procedure and stopped once procedure was over. The
cough score, secretion score, and physician satisfaction score were
decided separately by the bronchoscopist and an independent observer as
soon as the procedure was over, using the 100 mm visual analogue score
[11-13]. The best possible response was taken as 100 and the worse
possible finding was scored as 0. Scores were documented independently
and the average was taken as the final score.
Pauses in respiration, maximum drop of pulse rate and
maximum rise of pulse rate were also documented. Recovery time was
documented as time to regain full consciousness (in minutes) after the
end of bronchoscopy procedure. The stop watch readings was documented
once Ramsay score 01 was achieved. Monitoring and administration of
sedation were done by two residents, assisted by pediatric respiratory
nurse. The bronchoscopies were performed by experienced pediatricians.
Statistical analysis: Data were collected using a
pre-tested data collection sheet by principal investigator and data were
managed using Microsoft Excel. STATA 13 (Stata Corp., College Station,
TX, USA) was used for analysis. Time to achieve Ramsay score 3, visual
analogue scores (physician satisfaction, cough, secretion), additional
doses of midazolam, and time to achieve full recovery in two groups were
calculated and expressed as mean (SD). Differences were compared using
independent t test. In addition, Fisher’s exact test was used to compare
categorical variables. Intention-to-treat analysis was used. Statistical
significance was taken as P value less than 0.05.
Results
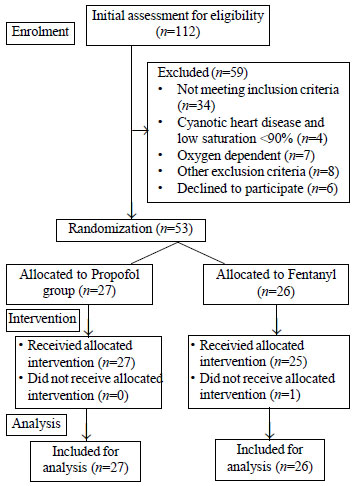
One hundred and twelve children were screened for
eligibility for the study over a duration of approximately six months.
After excluding 59 children, a total of 53 children were randomized; 27
in propofol arm and 26 in fentanyl arm (Fig. 1). Fifty two
children completed the study and one patient who was in fentanyl group,
sedation was not administered according to the protocol (Fig.
1). Table I shows the baseline characteristics of the
enrolled children. There were no significant differences between the two
groups.
 |
|
Fig. 1 Study flow diagram.
|
TABLE I Baseline Characteristics of Study Participants
|
Propofol arm |
Fentanyl arm |
|
n= 27 |
n= 26 |
|
Male: Female |
11: 16 |
17: 9 |
|
Age, y |
9.6 (3.4) |
8.9 (3.5) |
|
At baseline |
|
Oxygen saturation, % |
99.1 (1.5) |
99.1 (1.4) |
|
Pulse rate, per min |
94.7 (7.7) |
98.3 (7.2) |
|
Respiratory rate, per min |
20.9 (2.9) |
21.4 (2.6) |
|
Ramsay score |
1 |
1 |
|
All values are mean (SD), unless specified. |
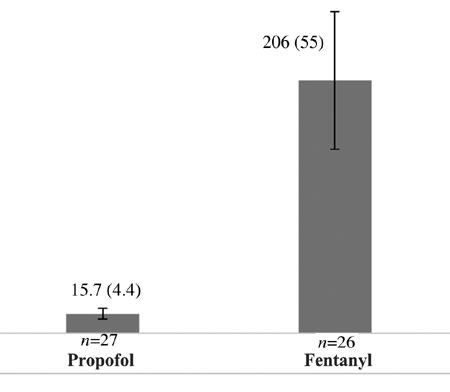
The mean (SD) time taken to achieve Ramsay score 3
was lower in the propofol arm than in the fentanyl arm [15.7 (4.4) s
vs 206 (55) s]; the mean difference (95% CI) was 190.3 (168.9,
211.6) s and it was statistically significant (P<0.001) (Fig.
2).
 |
|
Fig. 2 Mean (SD) time (s) taken to
achieve Ramsay score 3 in both arms.
|
The assessment of procedure related characteristics
(physician satisfaction score, cough score, and secretion score, need of
additional midazolam and number of additional midazolam) were
significantly better in propofol group (Table II). Safety
parameters were comparable between arms. The recovery time was
significantly quicker in propofol group (Table II).
TABLE II Secondary Outcomes in Children Undergoing Bronchoscopy
|
Propofol arm |
Fentanyl arm |
Mean (95% CI) |
P value |
|
n= 27 |
n= 26 |
difference |
|
|
Additional midazolam doses needed, no. |
11 |
25 |
Not applicable |
<0.001 |
|
Number of additional midazolam doses per patient, mean (SD) |
0.41 (0.5) |
1.96 (0.2) |
1.55 (1.33, 1.76) |
<0.001 |
|
Physician satisfaction: Visual analogue score, mean (SD) |
87 (12) |
54 (22) |
33.0 (23.2, 42.7) |
<0.001 |
|
Cough score: Visual analogue score, mean (SD) |
85 (10) |
56 (17) |
29.0 (21.3, 36.6) |
<0.001 |
|
Secretion score: Visual analogue score, mean (SD) |
89 (6) |
80 (11) |
9.0 (4.1, 13.8) |
0.001 |
|
Any pause in breathing, no. |
1 |
2 |
Not applicable |
0.507 |
|
Time taken to regain full consciousness, (min), mean (SD) |
7.7 (5.6) |
67 (27) |
59.3 (48.6, 69.9) |
<0.001 |
Two children from fentanyl group and one child from
propofol group had brief apneic episodes; however, they recovered with
stimulation without further intervention. In addition, 10 out of 27
children in propofol group complained of mild self-limiting burning
sensation at the site of administration but it was not observed at the
time of recovery.
Discussion
We performed this open label randomized controlled
trial to compare two sedative medications for conscious sedation in
paediatric bronchoscopy. Propofol had significantly faster sedation
induction time, less recovery time, less coughing, better physician
satisfaction and no differences in adverse effects as compared to
fentanyl.
Propofol slow bolus with or without infusion has been
previously studied and it was well tolerated in children [11,12]. The
drug has been approved for utilization in children [13]. However,
hypotension, bradycardia and apnoea were demonstrated in propofol
anaesthesia [12,14,15]. Similarly, fentanyl has been a well-established
medication in pediatric practice, especially for short procedures. It
has been used as bolus and infusions with minimal adverse effects though
post administration bradypnea and cardiovascular instability have been
reported [16]. Nevertheless, conscious sedation is effective and safer
than general anaesthesia for flexible bronchoscopy and level of sedation
can be monitored with Ramsay score [17].
Propofol and fentanyl have not been compared for
sedation for paediatric bronchoscopy in a trial. Lower induction time
for propofol in children was described by Rashed, et al. [7] in a
prospective study without comparative group. However, the induction time
was much higher than that of this study. Probably because deeper level
of anaesthesia was targeted [7]. Although sedation induction is not well
defined in children, fentanyl has quick action to achieve procedural
sedation [18]. In the field of paediatric gastroenterology,
non-anesthesiologists administer sedation commonly [19].
Physician satisfaction, level of cough, and level of
airway secretions are major parameters in assessing effectiveness of
sedation for bronchoscopy in many settings as a primary research tool
[9,20]. Physician satisfaction has been reported to be higher with
combinations of propofol/opioids and propofol/benzodiazepines than
propofol or volatile agents [9]. Cough response is much lower with
opioid-driven sedation than propofol [21-23]. We observed that propofol
arm performed better than fentanyl on these parameters.
Bradycardia and respiratory depression have been
reported with propofol, however, it is comparatively higher with
combination of sedatives [21,24,25]. Despite sedation, opioids may be
associated with higher pulse rate and respiratory depression [10,21,24].
Similar findings were observed in our study although none of the
children had significant adverse event. Mild self-limiting burning
sensation at injection site is a known untoward effect of propofol [26].
Recovery time is one of the determinants of duration
of hospital stay and duration of post procedure monitoring. Therefore,
it influences the utilization of resources and manpower in the
institution. Lower recovery time would improve cost effectiveness and
patient safety [17,22]. In our study, children receiving propofol had
faster recovery and shorter time of drowsiness, confirming observations
of earlier studies [7,12,21]. Fentanyl is considered to have a quicker
recovery time in comparison with other opioids [17].
In this experimental study, target level of
anaesthesia was lower. Therefore, induction time could have been
shorter. In addition, utilization of medication dose and top-up doses
could have been lower; all these could be reasons for lower adverse
effects and shorter recovery. Moreover, utilisation of solitary
medication in propofol arm could have led to better outcome.
A limitation of the study was its open-label design.
As propofol and fentanyl can easily be distinguished with external
appearance and having a subsequent infusion, therefore double dummy
technique could have been used to overcome the situation. The strength
of this study was a randomized control design with adequate sample size.
Our study suggest that propofol can be used safely and effectively by
well-trained pediatrician for flexible bronchoscopy in children. It
provides one more option of conscious sedation in practice of flexible
bronchoscopy in children.
To conclude, propofol may have shorter sedation
induction time, better procedure related satisfaction and quicker
recovery when used for conscious sedation in pediatric bronchoscopy.
Acknowledgements: Pediatric Respiratory nurse,
residents of Division of Pulmonology and staff of Pediatric day-care
services, All India Institute of Medical Sciences, New Delhi.
Contributors: PKGG: involved in developing
protocol, data collection, analysis and manuscript writing; KRJ:
involved in developing protocol and manuscript writing; JS: involved in
data analysis and manuscript writing; RL: involved in developing
protocol, data analysis and manuscript writing; RL,KRJ, SKK: revised it
critically for important intellectual content; SKK: involved in study
idea, protocol development; data collection, manuscript writing and will
act as guarantor for the study; all authors approved final version of
manuscript.
Funding: None; Competing interest: None
stated.
|
What is Already Known?
• Conscious sedation is increasingly being
utilized for flexible bronchoscopy in children.
• Combination of propofol and fentanyl is
better than volatile agents for pediatric bronchoscopy.
What This Study Adds?
• Propofol has shorter sedation induction
time, better procedure related satisfaction and quicker recovery
as compared to fentanyl in pediatric flexible bronchoscopy.
• Propofol is an effective and safe modality for conscious
sedation in pediatric bronchoscopy.
|
References
1. Dooms C, Seijo L, Gasparini S, Trisolini R, Ninane
V, Tournoy KG. Diagnostic bronchoscopy: state of the art. Eur Respir
Rev. 2010;19:229-36.
2. Brownlee KG, Crabbe DCG. Paediatric bronchoscopy.
Arch Dis Child. 1997;77:272-5.
3. McQuaid KR, Laine L. A systematic review and
meta-analysis of randomized, controlled trials of moderate sedation for
routine endoscopic procedures. Gastrointest Endosc. 2008;67:910-23.
4. Mahajan C, Dash HH. Procedural sedation and
analgesia in pediatric patients. J Pediatr Neurosci. 2014;9:1-6.
5. Jaggar SI, Haxby E. Sedation, anaesthesia and
monitoring for bronchoscopy. Paediatr Respir Rev. 2002;3:321-7.
6. Faulx AL, Vela S, Das A, Cooper G, Sivak MV,
Isenberg G, et al. The changing landscape of practice patterns
regarding unsedated endoscopy and propofol use: a national Web survey.
Gastrointest Endosc. 2005;62:9-15.
7. Hasan RA, Reddy R. Sedation with propofol for
flexible bronchoscopy in children. Pediatr Pulmonol. 2009;44: 373-8.
8. Patel KN, Simon HK, Stockwell CA, Stockwell JA,
DeGuzman MA, Roerig P-L, et al. Pediatric procedural sedation by
a dedicated nonanesthesiology pediatric sedation service using propofol.
Pediatr Emerg Care. 2009;25:133-8.
9. Chen L, Yu L, Fan Y, Manyande A. A comparison
between total intravenous anaesthesia using propofol plus remifentanil
and volatile induction/ maintenance of anaesthesia using sevoflurane in
children undergoing flexible fibreoptic bronchoscopy. Anaesth Intensive
Care. 2013;41:742-9.
10. Essler CN, Jo Grap M, Ramsay MA. Evaluating and
monitoring analgesia and sedation in the intensive care unit. Crit Care.
2008;12:S2.
11. Vardi A, Salem Y, Padeh S, Paret G, Barzilay Z.
Is propofol safe for procedural sedation in children? A prospective
evaluation of propofol versus ketamine in pediatric critical care. Crit
Care Med. 2002;30:1231-6.
12. Grendelmeier P, Tamm M, Pflimlin E, Stolz D.
Propofol sedation for flexible bronchoscopy: a randomised,
noninferiority trial. Eur Respir J. 2014 ;43:591-601.
13. Smith MC, Williamson J, Yaster M, Boyd GJC,
Heitmiller ES. Off-label use of medications in children undergoing
sedation and anesthesia. Anesth Analg. 2012;115:1148-54.
14. Robinson BJ, Ebert TJ, O’Brien TJ, Colinco MD,
Muzi M. Mechanisms whereby propofol mediates peripheral vasodilation in
humans. Sympathoinhibition or direct vascular relaxation?
Anesthesiology. 1997;86:64-72.
15. Dahan A, Nieuwenhuijs DJF, Olofsen E. Influence
of propofol on the control of breathing. Adv Exp Med Biol.
2003;523:81-92.
16. Krauss BS, Krauss BA, Green SM. Procedural
sedation and analgesia in children. N Engl J Med. 2014;371:91.
17. José RJ, Shaefi S, Navani N. Sedation for
flexible bronchoscopy: current and emerging evidence. Eur Respir Rev.
2013;22:106-16.
18. Usta B, Türkay C, Muslu B, Gözdemir M, Kasapoglu
B, Sert H, et al. Patient-controlled analgesia and sedation with
alfentanyl versus fentanyl for colonoscopy: a randomized double blind
study. J Clin Gastroenterol. 2011;45:e72-75.
19. Orel R, Brecelj J, Dias JA, Romano C, Barros F,
Thomson M, et al. Review on sedation for gastrointestinal tract
endoscopy in children by non-anesthesiologists. World J Gastrointest
Endosc. 2015;7:895–911.
20. Silvestri GA, Vincent BD, Wahidi MM, Robinette E,
Hansbrough JR, Downie GH. A phase 3, randomized, double-blind study to
assess the efficacy and safety of fospropofol disodium injection for
moderate sedation in patients undergoing flexible bronchoscopy. Chest.
2009;135:41-7.
21. Yoon HI, Kim J-H, Lee J-H, Park S, Lee C-T, Hwang
J-Y, et al. Comparison of propofol and the combination of
propofol and alfentanil during bronchoscopy: A randomized study. Acta
Anaesthesiol Scand. 2011;55: 104-9.
22. Wahidi MM, Jain P, Jantz M, Lee P, Mackensen GB,
Barbour SY, et al. American College of Chest Physicians consensus
statement on the use of topical anesthesia, analgesia, and sedation
during flexible bronchoscopy in adult patients. Chest. 2011;140:1342-50.
23. Schlatter L, Pflimlin E, Fehrke B, Meyer A, Tamm
M, Stolz D. Propofol versus propofol plus hydrocodone for flexible
bronchoscopy: a randomised study. Eur Respir J. 2011;38:529-37.
24. de Blic J, Marchac V, Scheinmann P. Complications
of flexible bronchoscopy in children: prospective study of 1,328
procedures. Eur Respir J. 2002;20:1271-6.
25. Somu N, Vijayasekaran D, Ashok TP, Balachandran
A, Subramanyam L. Flexible fibreoptic bronchoscopy in 582 children –
value of route, sedation and local anesthetic. Indian Pediatr.
1995;32:543-7.
26. Chidambaran V, Costandi A, D’Mello A. Propofol: A review of its
role in pediatric anesthesia and sedation. CNS Drugs. 2015;29:543-63.
|
|
|
 |
|

