|
|
|
Indian Pediatr 2017;54: 1054 -1055 |
 |
Neuromelioidosis Masquerading as Acute
Demyelinating Encephalomyelitis
|
|
Alok Shimee Ekka, Mohamed Mohideen and Sajith Kesavan
From Pediatric Intensive Care Unit, Kanchi Kamakoti CHILDS Trust
Hospital, Chennai.
Correspondence to: Dr. Alok Shimee Ekka, Kanchi Kamakoti CHILDS Trust
Hospital, 12 A, Nageswara road, Nungamakkam, Chennai 600034, India.
Email: ilovekorba08@gmail.com
Received: January 09, 2017;
Initial review completed : May 18, 2017;
Accepted: October 04, 2017.
|
Background: Neuromelioidosis is a rare
conduction, which is difficult to diagnose and treat. Case
characteristics: Preadolescent girl presenting with prolonged fever,
acute ascending paralysis and encephalopathy. Outcome:
Neuromelioidiosis was confirmed on brain biopsy culture. Patient
improved with an intensive antibiotic regimen. Message:
Neuromelioidosis can mimic acute demyelinating encephalomyelitis
clinically and radiologically.
Keywords: Brain biopsy, Febrile encephalopathy, Ring enhancing
lesion.
|
|
M
elioidosis is a tropical infection caused by
Burkholderia pseudomallei [1]. Encephalomyelitis like presentation
with quadriplegia and encephalopathy, evolving into focal suppurative
central nervous system lesions, is very rare. We report a
preadolescent girl with such a presentation.
Case Report
An 11-year old, previously healthy and
developmentally normal girl, presented with a history of fever for
twenty days and a left gluteal abscess that had been previously drained.
Histo-pathological examination of the excised sinus tract of the above
mentioned abscess revealed chronic inflammation. Pus culture and
GeneXpert MTB/RIF were negative.
She continued to have fever and developed rapidly
progressing ascending paralysis of all limbs with urinary retention.
Neurological examination showed neck rigidity, flaccid paralysis with
areflexia in both lower limbs and weakness of the upper limbs with
preserved deep tendon reflexes. Plantar reflexes were absent
bilaterally. Examination of the cranial nerves and sensory system were
normal. She was treated empirically with Meropenem, Vancomycin and
anti-tubercular therapy.
Her cerebrospinal (CSF) analysis revealed
lympho-cytic pleiocytosis (WBC-248 cells/µL, 92% lympho-cytes), elevated
proteins (69 mg/100 mL) and normal sugar. CSF AFB stain and GeneXpert
MTB/RIF were negative, and Adenosine deaminase levels were normal.
Hematological work-up revealed neutrophilc leuco-cytosis, high CRP and
normal renal and liver function. HIV serology was negative. Contrast MRI
of the brain and spine showed multiple asymmetric, non-enhancing T2
hyperintense lesions in the white matter of the brain and the entire
spinal cord, which strongly favoured a diagnosis of Acute Disseminated
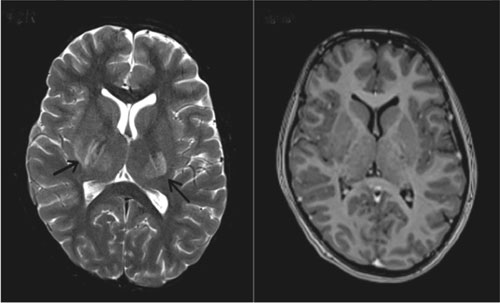
Encephalomyelitis (ADEM) (Fig. 1a).
 |
| (a) |
(b) |
|
Fig. 1 (a) Axial T2-weighted MRI of
the brain showing hyper intense lesions bilaterally in the basal
ganglia and thalamus (arrows) done on the day of admission; (b)
Axial T1-weighted post contrast MRI shows no focal enhancing
lesions.
|
Pulse methylprednisolone therapy was started for
ADEM. Since blood, urine and CSF cultures were sterile, ceftriaxone was
used as the sole antibiotics. Anti-tubercular treatment was continued.
Over the next few days, she developed altered sensorium. Plasmapheresis
was started on the 4th day after admission because of inadequate
response to steroids. Even after three cycles of plasma exchange, she
did not show signs of improvement and started having high-grade fever
spikes. Repeat MRI brain and spine showed complete clearing of the
spinal lesions, but new multiple ring-enhancing nodular lesions in both
cerebral hemispheres, making the diagnosis of ADEM unlikely. Therefore,
plasmapheresis and steroids were discontinued. Meropenem and vancomycin
were restarted after sending repeat cultures from CSF, blood and urine.
Empirical Amphotericin-B for fungal infection and Trimethoprim/Sulfamethoxazole
(TMP/SMX) for Toxoplasmosis were added. All cultures were still sterile.
CSF Galactomannan assay, India ink for Cryptococcus, Mycoplasma PCR and
GeneXpert MTB/RIF were negative. Serology for Brucella, Toxoplasma and
Mycoplasma were also negative. Repeat CSF examination continued to show
lymphocytic pleocytosis (WBC-270 cells/µL, 98% lymphocytes).
Due to continuing encephalopathy, a third MRI brain
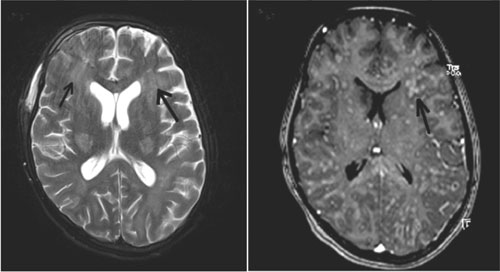
and spine was done on day 14, which revealed numerous small,
ring-enhancing lesions in both cerebral hemispheres, thalami, midbrain
and cerebellum (Fig. 2b). As the number of
lesions had increased significantly and the patient continued to be
encephalopathic, a brain biopsy (through right frontal craniotomy) was
per-formed. Histopathology showed chronic granulomatous inflammation.
Bacterial culture done by Vitek-2 (Biomerieux, France) from the biopsy
sample grew Burkholderia pseudomallei and a definitive diagnosis
of neuromeliodiosis was made. Ceftazidime and TMP/SMX were started
intravenously. After one week of therapy, she became afebrile and her
sensorium improved. At the end of 4 weeks she had significant
neurological improvement; she regained power in her lower limbs and was
interacting well with her parents. MRI brain showed a decrease in the
number of enhancing lesions. She was discharged with a plan of
eradication therapy with oral TMP-SMX and Doxycycline for one year. When
the child came for follow-up, she was neurologically normal. Work up for
immunodeficiency including serum immunuglobulins, flow cytometry and
Nitro blue tetrazolium test were normal during follow-up.
 |
| (a) |
(b) |
|
Fig. 2 (a) MRI brain on day 14 of
admission, axial T2 weighted image shows persisting hyperintense
lesions bilaterally in basal ganglia and new confluent
hyperintensities in the subcortical white matter in the
bilateral frontal and parietal regions (arrows); (b)
Axial T1-weighted post contrast MRI shows multiple small
nodular and ring-enhancing lesions (arrow).
|
Discussion
Melioidosis is endemic in South East Asia and
Northern Australia, and is under-diagnosed and under-reported in India
[2,3]. Neuromelioidosis is very rare with less
than 50 cases reported over the last 30 years [4]; however, mortality in
neuromelioidosis is high [5]. The most common presentation of
neuromelioidosis is mening-oencephalitis. It can also present as
cerebral abscess (ring enhancing lesions), myelitis, monoparesis,
paraparesis, cranial nerve palsies and can mimic Guillain-Barre syndrome
[5].
Culture represents the diagnostic gold standard for
melioidosis [1].
Treatment of neuromelioidosis includes parenteral antibiotics
Ceftazidime or Carbapenem plus TMP/SMX for a minimum of 4 weeks,
followed by oral eradication therapy with TMP/SMX plus/or
Doxycyline for a minimum of 6 to 12 months [6]. In the present case,
even though the presentation and radiological picture favored a
diagnosis of ADEM, there were atypical findings in the CSF, clinical
course and treatment response. Progression of neurological disease
despite immuno-therapy and persistence of fever prompted repeat CSF and
serial imaging, which led to reconsideration of the provisional
diagnosis of ADEM. Despite repeated negative cultures from blood and CSF
we were able to isolate the organism from the brain biopsy sample.
This case highlights the importance of serial brain
imaging and the utility of brain biopsy in cases where there is
inadequate response to empirical treatment regimes.
Acknowledgements: Dr Swetha Lakshmi Narla and Dr
Annapurneswari S, Department of Histopathology, Apollo Cancer
Institutes, Teynampet, Chennai, for reporting the histopathology slides
of the brain biopsy and providing pictures for the same; and Dr B
Chidambaram, Consultant Pediatric Neurosurgeon, KK CHILDS Trust
Hospital, Nungambakkam, Chennai, for performing the brain biopsy.
Contributors: ASE – Data collection, manuscript
preparation. MM – Manuscript editing. SK – Manuscript editing.
Funding: None. Conflict of interest: None
stated.
References
1. Limmathurotsakul D, Peacock SJ. Melioidosis: A
clinical overview. Bri Med Bull. 2011;99:125-39.
2. John TJ. Emerging and re-emerging bacterial
pathogens in India. Indian J Med Res. 1996;103:4-18.
3. Currie BJ, Jacups SP. Intensity of rainfall and
severity of melioidosis, Australia. Emerg Infect Dis. 2003;9:1538-42.
4. Muthusamy KA, Waran V, Puthucheary SD. Spectra of
central nervous system melioidosis. J Clin Neurosci. 2007;14:1213-5.
5. Currie BJ, Ward L, Cheng AC. The epidemiology and
clinical spectrum of melioidosis: 540 cases from the 20 year DARWIN
prospective study. PLoS Negl Trop Dis. 2010;4:e900.
6. Limmathurotsakul D, Chaowagul W, Wongsrikaew P,
Narmwong A, Day NP, Peacock SJ. Variable presentation of neurological
melioidosis in Northeast Thailand. Am J Trop Med Hyg. 2007;77:118-20.
|
|
|
 |
|

